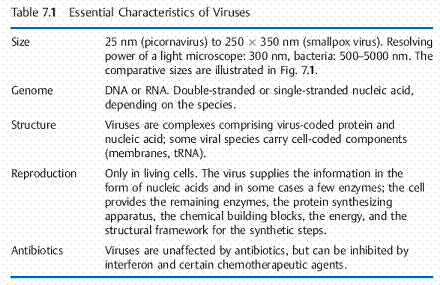
VIRUSES
Viruses are complexes consisting of protein and an RNA or DNA genome. They lack both cellular structure and independent metabolic processes. They replicate solely by exploiting living cells based on the information in the viral genome.
Viruses are autonomous infectious particles that differ widely from other microorganisms in a number of characteristics: they have no cellular structure, consisting only of proteins and nucleic acid (DNA or RNA). They have no metabolic systems of their own, but rather depend on the synthetic mechanism of a living host cell, whereby the viruses exploit normal cellular metabolism by delivering their own genetic information, i.e., nucleic acid, into the host cell. The host cell accepts the nucleic acid and proceeds to produce the components of new viruses in accordance with the genetic information it contains. One thus might call viruses “vagabond genes.” Viruses infect bacteria (so-called bacteriophages), plants, animals, and humans.

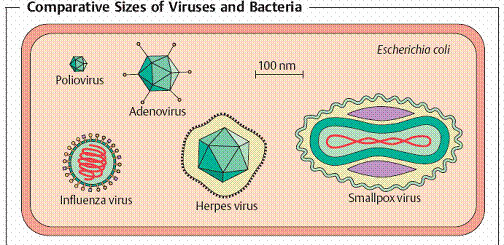
Morphology and Structure
A mature virus particle is also known as a virion. It consists of either two or three basic components:
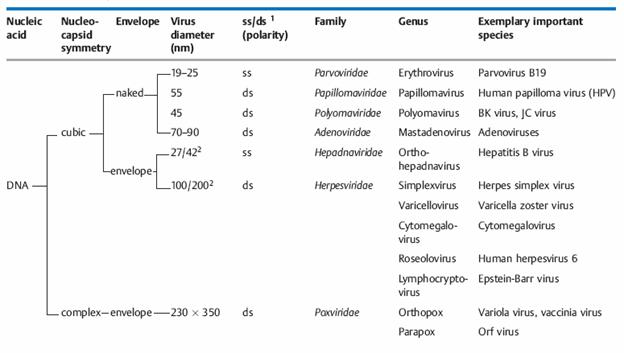
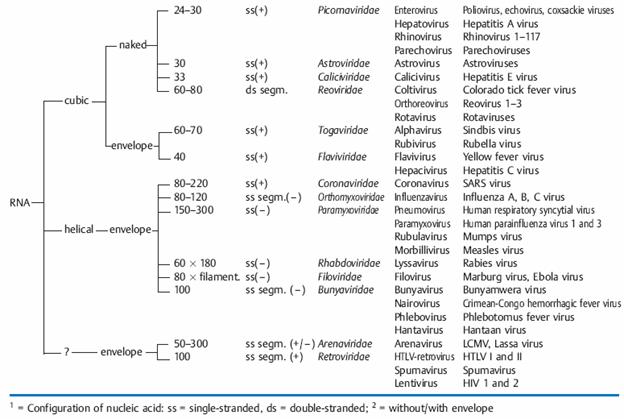
— A genome of DNA or RNA, double-stranded or single-stranded, linear or circular, and in some cases segmented. A single-stranded nucleic acid can have plus or minus polarity.
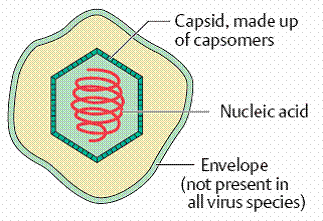
— The capsid, virus-coded proteins enclosing the nucleic acid of the virus and determining its antigenicity; the capsid can have a cubic (rotational), helical or complex symmetry and is made up of subunits called capsomers.
— In some cases an envelope ) that surrounds the capsid and is always derived from cellular membranes.
Genome: The viral genome is either DNA or RNA, and viruses are hence categorized as DNA or RNA viruses. The nucleic acid of DNA viruses is usually double-stranded (ds) and linear or circular depending on the family; the nucleic acid of RNA viruses is usually single-stranded (ss), with the exception of the reoviruses, and is also segmented in a number of virus families. Viruses with ssRNA are divided into two groups: if the RNA of the genome has the same polarity as the viral mRNA and can thus function directly as messenger RNA it is called a plus-strand (or positive-strand) or “sense” RNA strand and these viruses are sense or plus-strand viruses. If the genome RNA has the polarity opposite to that of the mRNA, and therefore cannot be translated into proteins until it has first been transcribed into a complementary strand, it is called a minus-strand (or negative-strand) or “antisense” RNA strand and the viruses are antisense or minus-strand viruses.
Capsid: The capsid is the “shell” of virus-coded protein that encloses the nucleic acid and is more or less closely associated with it. The combination of these two components is often termed the nucleocapsid, especially if they are closely associated as in the myxoviruses. The capsid is made up of subunits, the capsomers, the number of which varies but is specific and constant for each viral species. These are spherical or cylindrical structures composed of several polypeptides. The capsid protects the nucleic acid from degradation. In all except enveloped viruses, it is responsible for the attachment of the viruses to the host cell (“adsorption,” see the chapter on replication, and determines specific viral antigenicity.
Envelope: The envelope, which surrounds the capsid in several virus families, is always dependent on cellular membranes (nuclear or cell membrane, less frequently endoplasmic reticulum). Both cell-coded and viral proteins are integrated in the membrane when these elements are transformed into the envelope, frequently in the form of “spikes” (or peplomers. Enveloped viruses do not adsorb to the host cell with the capsid, but rather with their envelope. Removing it with organic solvents or detergents reduces the infectivity of the viruses (“ether sensitivity”).
Various enzymes: Viruses require a number of different enzymes depending on genome type and mode of infection. In several virus species enzymes are a component of the virus particle, for example the neuraminidase required for invasion and release of myxoviruses. Other examples include nucleic acid polymerases such as the RNA-dependent RNA polymerases in antisense viruses, the DNA polymerases in smallpox viruses and the RNA-dependent DNA polymerase (“reverse transcriptase”) in hepatitis B viruses and retroviruses .
Hemagglutinin: Some viruses (above all myxoviruses and paramyxoviruses) are capable of agglutinating various different human or animal erythrocytes. These viruses bear a certain surface protein (hemagglutinin) in their envelope that enables them to do this. The hemagglutination phenomenon can be made use of for quantitative viral testing or—in the hemagglutination inhibition test—for virus identification and antibody identification. In biological terms, hemagglutinin plays a decisive role in adsorption and penetration of the virus into the host cell.
Structural Patterns:
Cubic symmetry (rotational symmetry): Viruses with rotational symmetry are icosahedrons (polyhedrons with 20 equilateral triangular faces). The number of capsomers per virion varies from 32 to 252 and depends on the number of capsomers (two to six) making up one side of the equilateral triangle. The capsomers in a virion need not all be the same, either in their morphology, antigen make-up or biological properties. Purified icosahedral viruses can be crystallized, so that images of them can be obtained using the methods of radiocrystallography. A number of virus images have been obtained with this method at a resolution of 2 A.
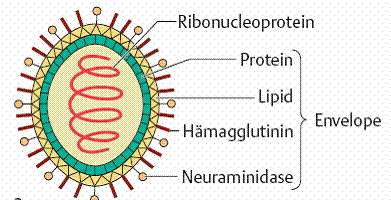
Helical symmetry: Helical symmetry is present when one axis of a capsid is longer than the other. The nucleic acid and capsid protein are closely associated in the ribonucleoprotein (RNP), in which the protein is tightly arrayed around the nucleic acid strand. This RNA-protein complex is known as the nucleocapsid, which takes the form of a helix inside the viral envelope.
Complex symmetry: Complex structural patterns are found in bacteriophages and the smallpox virus . T bacteriophages, for example, have an icosahedral head containing the DNA and a tubelike tail through which the DNA is injected into the host cell.
Classification
The taxonomic system used for viruses is artificial (i.e., it does not reflect virus evolution) and is based on the following morphological and biochemical criteria:
— Genome: DNA or RNA genome (important basic differentiation of virus types!) as well as configuration of nucleic acid structure: single-stranded (ss) or double-stranded (ds); RNA viruses are further subclassified according to plus and minus polarity.
— Capsid symmetry: cubic, helical, or complex symmetry.
— Presence or absence of an envelope.
— Diameter of the virion, or of the nucleocapsid with helical symmetry.
Virus Classification:
Virus classification involves naming and placing viruses into a taxonomic system. Like the relatively consistent classification systems seen for cellular organisms, virus classification is the subject of ongoing debate and proposals. This is largely due to the pseudo-living nature of viruses, which are not yet definitively living or non-living. As such, they do not fit neatly into the established biological classification system in place for cellular organisms, such as plants and animals, for several reasons.
Virus classification is based mainly on phenotypic characteristics, including morphology, nucleic acid type, and mode of replication, host organisms, and the type of disease they cause. A combination of two main schemes is currently in widespread use for the classification of viruses. David Baltimore, a Nobel Prize-winning biologist, devised the Baltimore classification system, which places viruses into one of seven groups. These groups are designated by Roman numerals and separate viruses based on their mode of replication, and genome type. Accompanying this broad method of classification are specific naming conventions and further classification guidelines set out by the International Committee on Taxonomy of Viruses.
Baltimore classification is a classification system which places viruses into one of seven groups depending on a combination of their nucleic acid (DNA or RNA), strandedness (single-stranded or double-stranded), and method of replication. Other classifications are determined by the disease caused by the virus or its morphology, neither of which are satisfactory due to different viruses either causing the same disease or looking very similar. In addition, viral structures are often difficult to determine under the microscope. Classifying viruses according to their genome means that those in a given category will all behave in a similar fashion, offering some indication of how to proceed with further research. Viruses can be placed in one of the seven following groups:
I: dsDNA viruses (e.g. Adenoviruses, Herpesviruses, Poxviruses)
II: ssDNA viruses (+)sense DNA (e.g. Parvoviruses)
III: dsRNA viruses (e.g. Reoviruses)
IV: (+)ssRNA viruses (+)sense RNA (e.g. Picornaviruses, Togaviruses)
V: (-)ssRNA viruses (-)sense RNA (e.g. Orthomyxoviruses, Rhabdoviruses)
VI: ssRNA-RT viruses (+)sense RNA with DNA intermediate in life-cycle (e.g. Retroviruses)
VII: dsDNA-RT viruses (e.g. Hepadnaviruses)
DNA viruses
Group I: viruses possess double-stranded DNA and include such virus families as Herpesviridae (examples like HSV1 (oral herpes), HSV2 (genital herpes), VZV (chickenpox), EBV (Epstein-Barr virus), CMV (Cytomegalovirus)), Poxviridae (smallpox) and many tailed bacteriophages. The mimivirus was also placed into this group.
Group II: viruses possess single-stranded DNA and include such virus families as Parvoviridae and the important bacteriophage M13.
|
Virus Family |
Examples (common names) |
Virion- naked/enveloped |
Capsid Symmetry |
Type of nucleic acid |
|
1.Adenoviridae |
Adenovirus |
Naked |
Icosahedral |
ds |
|
2.Papovaviridae |
Papillomavirus |
Naked |
Icosahedral |
ds circular |
|
3.Parvoviridae |
Parvovirus B19 |
Naked |
Icosahedral |
ss |
|
4.Herpesviridae |
Herpes simplex virus, varicella-zoster virus, cytomegalovirus, Epstein-Barr virus |
Enveloped |
Icosahedral |
ds |
|
5.Poxviridae |
Smallpox virus, vaccinia virus |
Complex coats |
Complex |
ds |
|
6.Hepadnaviridae |
Hepatitis B virus |
Enveloped |
Icosahedral |
circular, partially ds |
|
7.Polyomaviridae |
Polyoma virus; JC virus (progressive multifocal leucoencephalopathy) |
Naked |
Icosahedral |
ds circular |
RNA viruses
Group III: viruses possess double-stranded RNA genomes, e.g. rotavirus. These genomes are always segmented.
Group IV: viruses possess positive-sense single-stranded RNA genomes. Many well known viruses are found in this group, including the picornaviruses (which is a family of viruses that includes well-known viruses like Hepatitis A virus, enteroviruses, rhinoviruses, poliovirus, and foot-and-mouth virus), SARS virus, hepatitis C virus, yellow fever virus, and rubella virus.
Group V: viruses possess negative-sense single-stranded RNA genomes. The deadly Ebola and Marburg viruses are well known members of this group, along with influenza virus, measles, mumps and rabies.
|
Virus Family |
Examples (common names) |
Virion- naked/enveloped |
Capsid Symmetry |
Type of nucleic acid |
|
1.Reoviridae |
Reovirus, Rotavirus |
Naked |
Icosahedral |
ds |
|
2.Picornaviridae |
Enterovirus, Rhinovirus, Hepatovirus, Cardiovirus, Aphthovirus, Parechovirus, Erbovirus, Kobuvirus, Teschovirus |
Naked |
Icosahedral |
ss |
|
3.Caliciviridae |
Norwalk virus, Hepatitis E virus |
Naked |
Icosahedral |
ss |
|
4.Togaviridae |
Rubella virus |
Enveloped |
Icosahedral |
ss |
|
5.Arenaviridae |
Lymphocytic choriomeningitis virus |
Enveloped |
Complex |
ss |
|
6.Retroviridae |
HIV-1, HIV-2, HTLV-I |
Enveloped |
Modified Icosehedral |
ss |
|
7.Flaviviridae |
Dengue virus, Hepatitis C virus, Yellow fever virus |
Enveloped |
Complex |
ss |
|
8.Orthomyxoviridae |
Influenzavirus A, Influenzavirus B, Influenzavirus C, Isavirus, Thogotovirus |
Enveloped |
Helical |
ss |
|
9.Paramyxoviridae |
Measles virus, Mumps virus, Respiratory syncytial virus |
Enveloped |
Helical |
ss |
|
10.Bunyaviridae |
California encephalitis virus, Hantavirus |
Enveloped |
Helical |
ss |
|
11.Rhabdoviridae |
Rabies virus |
Enveloped |
Helical |
ss |
|
12.Filoviridae |
Ebola virus, Marburg virus |
Enveloped |
Helical |
ss |
|
13.Coronaviridae |
Corona virus |
Enveloped |
Complex |
ss |
|
14.Astroviridae |
Astrovirus |
Naked |
Icosahedral |
ss |
|
15.Bornaviridae |
Borna disease virus |
Enveloped |
Helical |
ss |
Reverse transcribing viruses
Group VI: viruses possess single-stranded RNA genomes and replicate using reverse transcriptase. The retroviruses are included in this group, of which HIV is a member.
Group VII: viruses possess double-stranded DNA genomes and replicate using reverse transcriptase. The hepatitis B virus can be found in this group.
Viral Infection
When a virus particle enters a cell and begins to reproduce itself, this is called a viral infection. The virus is usually very, very small compared to the size of a living cell. The information carried in the virus's DNA allows it to take over the operation of the cell, converting it to a factory to make more copies of it. For example, the polio virus can make over one million copies of itself inside a single, infected human intestinal cell.
The origins and evolution of the viruses are still largely in the dark. In contrast to the taxonomic systems used to classify the higher forms of life, we are therefore unable to classify viruses in such evolutionary systems. An international nomenclature committee groups viruses according to various criteria and designates these groups, analogously to the higher forms, as families, genera, and species. Despite this element of “artificiality” in the system nowin use, the groups appear to make biological sense and to establish order in the enormous variety of known viruses.
Viruses can be spread in the following exemplar ways
n Airborne - Viruses that infect their hosts from the open air
n Blood Borne - Transmission of the virus between organisms when infected blood enters an organisms circulatory system
n Contamination - Caused from the consumption of materials by organisms such as water and food which have viruses within
n Therefore viruses have many means of getting transmitted from one organism to another.
LIFE CYCLE
All viruses only exist to make more viruses. With the possible exception of bacterial viruses, which can kill harmful bacteria, all viruses are considered harmful, because their reproduction causes the death of the cells which the viruses entered. If a virus contains DNA, it inserts its genetic material into the host cell's DNA. If the virus contains RNA, it must first turn its RNA into DNA using the host cell's machinery, before inserting it into the host DNA. Once it has taken over the cell, viral genes are then copied thousands of times, using the machinery the host cell would ordinarily use to reproduce its own DNA. Then the host cell is forced to encapsulate this viral DNA into new protein shells; the new viruses created are then released, destroying the cell.
All living things are susceptible to viral infections including plants, animals,
or bacteria can all be infected by a virus specific for that type of organism.
Moreover, within an individual species there may be a hundred or more different
viruses which can infect that species alone. There are viruses which infect only
humans (for example, smallpox), viruses which infect humans and one or two
additional kinds of animals (for example, influenza), viruses which infect only
a certain kind of plant (for example, the tobacco mosaic virus), and some
viruses which infect only a particular species of bacteria (for example, the
bacteriophage which infects E. coli).
Taxanomy:
Nomenclautre for virus is initially given on the basis of their host of infection. For example, animal viruses, plant viruses and bacteriophage etc., By the 1960s, however, virologists realized that some viruses can affect more than a single organ system or host. Classifying a virus as a brain virus or a skin virus or as one that infects a particular organism was no longer adequate.
A new classification system was needed. A committee, later named the International Committee on Taxonomy of Viruses (ICTV), set about that task at the International Congress of Microbiology in Moscow in 1966. The system they proposed is not the traditional Linnaean scheme used for all cellular organisms. The ICTV scheme has only three hierarchical levels-family (including some subfamilies), genus, and species. Family names all end in viridae. Genus names end in virus. Species names are English words.
Using the ICTV system, the virus that causes AIDS is classified as:
Family: Retroviridae
Genus: Lentivirus
Species: HIV (Human Immunodeficiency Virus)
Family names are often converted into English. Thus, the Retroviridae are called retroviruses. This practice removes the distinction between family and genus.
Acquired Immunodeficiency Syndrome (AIDS)
It is a remarkable and tragic fact that although AIDS was recognized as a distinct disease entity as recently as the 1980s, in this brief period it has become one of the most devastating afflictions in the history of mankind. AIDS is caused by infection with the human immunodeficiency virus (HIV). It is estimated that there are more than 42 million HIV-infected individuals in the world, more than 21 million deaths attributable to this disease, and more than 3 million deaths annually. The infection continues to spread, especially in Africa and Asia; and in some countries in Africa, more than 30% of the population has been infected with HIV. The following section describes the important features of HIV, how it infects humans, and the disease it causes. The section concludes with a brief discussion of the current status of therapy and vaccine development.
The Human Immunodeficiency Virus (HIV)
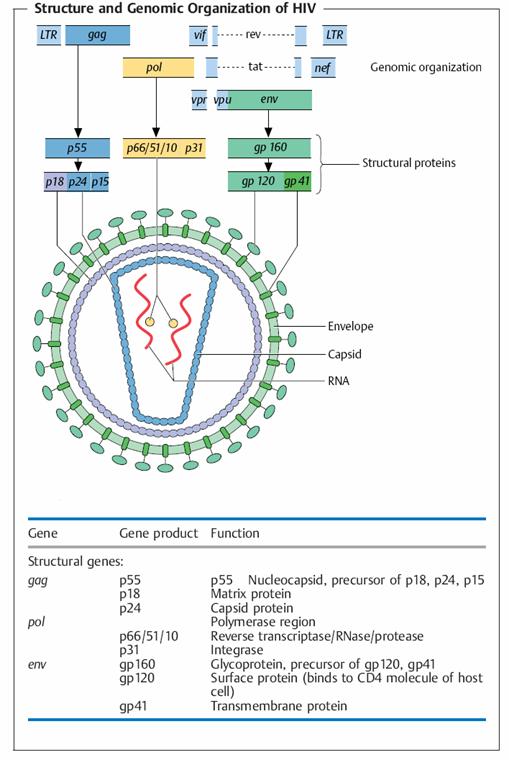
HIV is a retrovirus that infects cells of the immune system, mainly CD4* T lymphocytes, and causes progressive destruction of these cells. An infectious HIV particle consists of two RNA strands within a protein core, surrounded by a lipid envelope derived from infected host cells but containing viral proteins. The viral RNA encodes structural proteins, various enzymes, and proteins that regulate transcription of viral genes and the viral life cycle.
The life cycle of HIV consists of the following sequential steps: infection of cells, production of viral DNA and its integration into the host genome, expression of viral genes, and production of viral particles. HIV infects cells by virtue of its major envelope glycoprotein, called gpl20 (for 120 kD glycoprotein), binding to CD4 and particular chemokine receptors (CXCR4 and CCR5) on human cells.
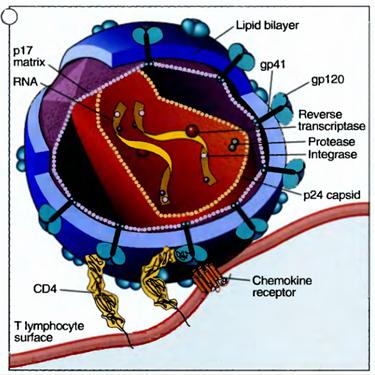
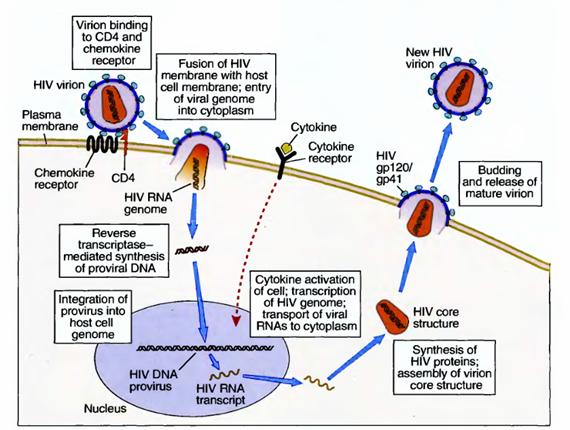
Therefore, the virus can efficiently infect only cells expressing CD4 and these chemokine receptors. The major cell type that may be infected by HIV is the CD4+ T lymphocyte, but macrophages and dendritic cells are also infected by the virus. Different cell populations may use different chemokine receptors to bind slightly different strains of the virus. After binding to cellular receptors, the viral membrane fuses with the host cell membrane and the virus enters the cell's cytoplasm. Here the virus is uncoated by viral protease and its RNA is released. A DNA copy of the viral RNA is synthesized by the virus's reverse transcriptase enzyme (a process that is characteristic of all retroviruses), and the DNA integrates into the host cell's DNA by the action of the integrase enzyme. The integrated viral DNA is called a provirus. If the infected T cell, macrophage, or dendritic cell is activated by some extrinsic stimulus, such as another infectious microbe, the cell responds by turning on the transcription of many of its own genes and often by producing cytokines. An unfortunate consequence of this normal response is that the cytokines, and the process of cellular activation itself, may also activate the provirus, leading to production of viral RNAs and then proteins. The virus is now able to form a core structure, which migrates to the cell membrane, acquires a lipid envelope from the host, and is shed as an infectious viral particle, ready to infect another cell. It is possible that the integrated HIV provirus remains latent within infected cells for months or years, hidden from the patient's immune system.
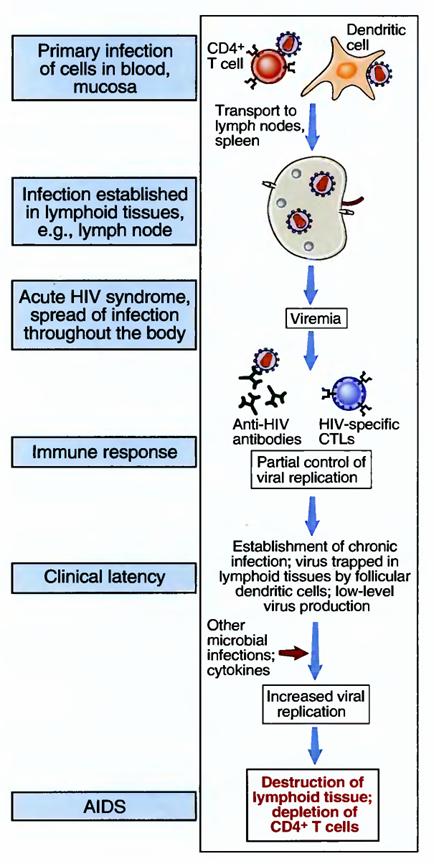
Most cases of AIDS are caused by HIV-1. A related virus, HIV-2, causes some cases of the disease. Pathogenesis of AIDS HIV establishes a latent infection in cells of the immune system and may be reactivated to produce infectious virus. This viral production leads to death of infected cells, as well as to death of uninfected lymphocytes, subsequent immune deficiencies, and clinical AIDS. HIV infection is acquired by sexual intercourse, contaminated needles used by intravenous drug users, transplacental transfer, or transfusion of infected blood or blood products. After infection there may be a brief, acute viremia, when the virus is detected in the blood, and the host may respond as in any mild viral infection. The virus infects CD4* T cells, dendritic cells, and macrophages in the blood, sites of entry through epithelia, and, most of all, lymphoid organs such as lymph nodes. Dendritic cells may capture the virus as it enters through epithelia and transport it to peripheral lymphoid organs, where it infects T cells. The integrated provirus may be activated in infected cells, as described previously, leading to production of viral particles and spread of the infection. During the course of HIV infection, the major source of infectious viral particles is activated CD4+ T cells; dendritic cells and macrophages are reservoirs of infection.
The depletion of CD4+ T cells after HIV infection is due to a cytopathic effect of the virus, resulting from production of viral particles, as well as death of uninfected cells. Active viral gene expression and protein production may interfere with the synthetic machinery of the T cells. Therefore, infected T cells in which the virus is replicating are killed during this process. The loss of T cells during the progression to AIDS is much greater than the numbers of infected cells. The mechanism of this T cell loss remains poorly defined. One possibility is that T cells are chronically activated, perhaps by infections that are common in these patients, and the chronic stimulation culminates in apoptosis, by the pathway called activation-induced cell death.
Other infected cells, such as dendritic cells and macrophages, may also die, resulting in destruction of the architecture of lymphoid organs. Many studies have suggested that immune deficiency results from various functional abnormalities in T lymphocytes and other immune cells, in addition to destruction of these cells. However, the significance of these functional defects has not been established, and loss of T cells remains the most reliable indicator of disease progression.
Clinical Features of HIV Infection and AIDS
The clinical course of HIV infection is characterized by several phases, culminating in immune deficiency. Early after HIV infection, patients may experience a mild acute illness with fever and malaise, correlating with the initial viremia. This illness subsides within a few days, and the disease enters a period of clinical latency. During this latency, there is usually a progressive loss of CD4+ T cells in lymphoid tissues and destruction of the architecture of the lymphoid tissues. Eventually, the blood CD4+ T cell count begins to decline, and when the count falls below 200 per mm3 (the normal being about 1500 cells per mm3), patients become susceptible to infections and are said to be suffering from AIDS.
The clinical and pathologic manifestations of full-blown AIDS are primarily the result of increased susceptibility to infections and some cancers, as a consequence of immune deficiency. Patients are often infected by intracellular microbes, such as viruses, Pneumocystis carinii, and atypical mycobacteria, all of which are normally combated by T cell—mediated immunity. Many of these microbes are present in the environment, but they do not infect healthy individuals with intact immune systems.
Because these infections are seen in immuno-deficient individuals, in whom the microbes have an opportunity to establish infection, these types of infections are said to be "opportunistic." Many of the opportunistic infections are caused by viruses, such as cytomegalovirus. AIDS patients show defective cytolytic T lymphocyte (CTL) responses to viruses, even though HIV does not infect CD8+ T cells. It is believed that the defective CTL responses are because CD4* helper T cells (the main targets of HIV) are required for full CD8+ CTL responses against many viral antigens. AIDS patients are at increased risk for infections by extracellular bacteria, likely because of impaired helper T cell-dependent antibody responses to bacterial antigens. Patients also become susceptible to cancers that are caused by oncogenic viruses. The two most common types of cancers are В cell lymphomas, caused by the Epstein-Barr virus, and a tumor of small blood vessels that is called Kaposi's sarcoma and is caused by a herpesvirus. AIDS patients with advanced disease often suffer from a wasting syndrome with a significant loss of body mass, due to altered metabolism and reduced caloric intake. Some AIDS patients develop dementia, believed to be caused by infection of macrophages (microglia) in the brain.
The immune response to HIV is ineffective in controlling spread of the virus and its pathologic effects. Infected individuals produce antibodies and CTLs against viral antigens, and the responses help to limit the early acute HIV syndrome. But these immune responses usually do not prevent chronic progression of the disease. Antibodies against envelope glycoproteins, such as gpl20, may be ineffective because the virus rapidly mutates the region of gpl20 that is the target of most antibodies. CTLs are often ineffective in killing infected cells because the virus inhibits the expression of class I MHC molecules by the infected cells. Immune responses to HIV may paradoxically promote spread of the infection. Antibody-coated viral particles may bind to Fc receptors on macrophages and follicular dendritic cells in lymphoid organs, thus increasing virus entry into these cells and creating additional reservoirs of infection. If CTLs are able to lyse infected cells, this may result in release of viral particles and infection of more cells. And, of course, by infecting and thereby interfering with the function of immune cells, the virus is able to prevent its own eradication.
Laboratory diagnosis:
The following diagnostic tools are currently available for confirmation of an HIV infection:
HIV antibody detection:
EIA screening tests are now available using genetically engineered or synthesized viral antigens (first to third generation of screening tests). Every positive result requires confirmation by an alternative test. The fourth-generation screening tests simultaneously detect antibodies to HIV 1 and 2 and p24 antigen (combination test) and are thus capable of detecting primary infections that are still antibody-negative.
HIV antigen detection:
In this test, a viral protein is detected in serum, usually capsid protein p24. The p24 antigen is detectable in serum as early as two weeks after infection and disappears again after eight to 12 weeks. Following a clinically stable latency period, HIV antigen can become detectable months or years later (transitory or persistent). This renewed appearance of HIV antigen is usually followed by manifest AIDS and is therefore a negative prognostic sign.
Rapid HIV test:
Antibody-based tests are available for rapid diagnosis in medical practices, hospitals, and health centers. Their specifications are equivalent to the third-generation screening tests.
PCR:
The most important application of the polymerase chain reaction today is to determine the so-called viral load, whereby a commercially available quantitative RT-PCR (reverse transcriptase PCR) is used to determine the number of viral RNA molecules per ml of blood, taking into account the added standard amounts of HIV RNA (quantification standard).
This test provides a prognostic estimate of how great the risk of progression to AIDS is (manifestation of an AIDS-defining disease). It can also be used to monitor the success of therapy with RT and protease inhibitors. The following HIV diagnostic procedure is now recommended: an HIV antibody screening test should first be performed to diagnose an HIV infection. If the test result is positive, a second serum specimen should be tested to confirm the result and exclude confusion of sera. If the initial screening test is negative, but a (primary) HIV infection is justifiably suspected, HIV antigen can be tested, for instance using the combination test.
Therapy and Vaccination Strategies
The current treatment of AIDS is aimed at controlling replication of HIV and the infectious complications of the disease. Cocktails of drugs that block the activity of the viral reverse transcriptase, protease, and integrase enzymes are now being administered early in the course of the infection, with considerable benefit. This treatment, called "highly active antiretroviral therapy (HAART)," is expensive, and its long-term efficacy is not known. The virus is capable of mutations that may render it resistant to these drugs, and the drug treatments do not eradicate reservoirs of latent virus.
Three classes of substances are available for HIV therapy:
— Nucleosidic (or nucleotidic) reverse transcriptase inhibitors (NRTI) (for example: azidothymidine, AZT; lamivudine, 3TC; didanosine, ddI, etc.). These are nucleoside analogs that bind to the active center of the enzyme are integrated in the DNA strands, resulting in “chain termination.”
— Nonnucleosidic reverse transcriptase inhibitors (NNRTI) (for example: efavirenz, EFV; nevirapine, NVP, etc.). This class of substances also inhibits the production of viral cDNA by reverse transcriptase, but does not prevent viral production by infected cells.
— Protease inhibitors (PI) (for example: indinavir, IDV; ritonavir, RTV; saquinavir, SQV, etc.): PIs inhibit viral protease and thus viral maturation.
To avoid development of resistant HIV variants, a combination of at least three drugs from at least two substance classes is usually administered. The following combinations are currently established practice:
a) One PI and two NRTIs
b) One NNRTI and two NRTIs
c) Two PIs and one or two NRTIs
d) One PI and one NNRTI, alternatively with one or two NRTIs as well;
e) Three NRTIs
a) and b) appear to produce the best long-term results.
The control of HIV worldwide will require the development of effective vaccines. A successful vaccine will likely have to induce an innate immune response, high titers of neutralizing antibodies, a strong T cell response, as well as mucosal immunity. An additional challenge is to be able to protect against all subtypes of HIV Early efforts focused on gpl20 as an immunogen, but these were largely unsuccessful. More recent attempts have involved combinations of DNA immunization and recombinant poxviruses encoding several different HIV proteins. It will take years to judge the effectiveness of new vaccines in clinical trials.
By following regular monitoring of blood transfusions, using sterile needles, avoiding drug abuses and safe sex, to the large extend HIV infection might be prevented from spreading.
Prions:
Prions for “proteinaceous infectious particles” are thought to cause about a dozen fatal degenerative disorders of the central nervous system of humans and other animals. Prions are unique among infectious agents beause they contain no nucleic acid; they are composed exclusively of protein.
The existence of an infectious agent composed only of protein seems to defy scientific dogma. First a prion does not replicate itself from scratch. It causes another protein to change its shape and thereby become a prion. A prion protein designated PrPsc is an altered form of a normal host protein (PrP). A prion has the capacity to change the secondary structure of a molecule of PrP so that it, too, becomes a prion. The secondary structure PrP is largely made up of alpha helices. A prion is largely made up of beta sheets. When a prion enters host it forms more prions from the cell’s supply of PrP. Prions accumulate, aggregate into fibrils and cause disease.
Stanley Prusiner, who did most of the work on prions, was awarded the Nobel prize in 1998. Many virologists, however, are still not convinced that prions alone cause these disease-they suspect that a still-undiscovered virus participates.
The evidence indicates that prions are protein molecules that cause degenerative central nervous system (CNS) diseases such as Creutzfeldt-Jakob disease, kuru, scrapie in sheep, and bovine spongiform encephalopathy (BSE) (general term: transmissible spongiform encephalopathies [TSE]).
Viroids:
A viroid is a circular molecule of ssRNA without a capsid. Viroids cause several economically important diseases of plants, including citrus exocortis. One such disease, potato spindle tuber, causes the edible part of the potato(the tuber) to become long and pointed. It also stunts the growth of tomato plants. As few as 10 molecules of the viroid are enough to infect a potato plant. Many more virions are needed to cause most plant disease.
A viroid is only about one-tenth the size of the small plant-virus. Spindle tuber viroid is composed of only 359 nucleotides, about a third as many as make up an ordinary-sized gene. How such a small molecule can cause disease, remains a mystery. It seems unlikely that the viroid encodes a protein that causes disease. More probably it interacts directly with the host genome, causing disease by changing the expression of the host genes. Viroids may be bits of mRNA from plant genes that have escaped during normal processing.