TRANSLATION
Translation or protein synthesis is a process in which the genetic information present in m-RNA in the form of triplet codes (codons) directs the sequence of aminoacids in protein. In otherwords, Translation is a series of events that converts the language of the genes, sequences of nucleotides into the language of polypeptides, sequence of aminoacids.
Factors required for Translation:
Following components play vital role in translation. They are
i) RNAs
ii) Ribosomes
iii) Translational factors
i) RNAs:
Mainly there are three types of RNAs, namely m-RNAs, r-RNAs and t-RNAs.
a) m-RNAs:
m-RNA is a class of RNA molecules each of it, is complementary to one strand of cell DNA and transfer early genetic message in the form of triplet codon from DNA to ribosome i.e. site of protein synthesis. Since, this class of RNA serves as messenger of information from DNA to ribosome, they are called as messenger RNA (m-RNAs).
m-RNA is a polymer of ribonucleotide where the ribonucleotides are linked by 3ΰ5 phosphodiester bonds. It is synthesized by RNA polymerase from the DNA template by transcriptional process.
Structural features:
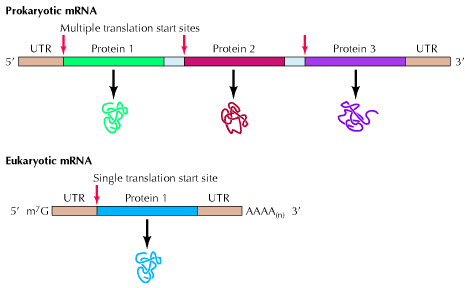
Prokaryotic m-RNA:
Size of m-RNA molecule is variable depending on the polypeptide chain products whose message it carries. Prokaryotic m-RNAs are polycistronic in nature i.e. they are polygenic messengers. It is a template for several polypeptide chains. For example a single m-RNA molecule codes for three specific enzymes to synthesis aminoacid tryptophan. In polycistronic m-RNA, the individual cistrons may be separated by intercistroonic sequences called spacers. The 5-end of the m-RNA may contain a sequence that is never translated into protein. These are called leader sequences or 5-untranslated region. A similar sequence is called 3-untranslated sequence may occur at the 3-end of the m-RNA. The life time of prokaryotic m-RNA is short compared to that of eukaryotic m-RNA.

In prokaryotes, the primary m-RNA transcript provides the functional m-RNA ready for translation. It has recently being found that towards the 5-end of m-RNA, there is a region of 20 or 30 nucleotides before initiation codon AUG is reached. This is nothing but the linear sequence contains the nucleotide sequence which is responsible for the interaction of the m-RNA with 30S subunit. It is known as Shine-Dalgarno (SD) sequence.

It has been showed that SD sequence binds to the complementary sequence at the 3-end of the 16S-rRNA of 30S subunit of ribosome to position the m-RNA correctly to start initiation.
Eukaryotic m-RNA:
Eukaryotic m-RNA is synthesized by RNA polymerase II. Eukaryotic m-RNAs are monocistronic in nature i.e. they are the template for the synthesis of a single polypeptide chain or it can be said that they carry codons only to code for single polypeptide chain. It has 7-methyl guanosine as a cap at its 3-end. It has poly A tail as a tail at 3-end. Exons and introns are present in it. In eukaryotes, the primary m-RNA transcript is not a functional component which is referred as heterogeneous nuclear RNA. The lifetime of eukaryotic m-RNA is greater than prokaryotic m-RNA. Kozak sequence at 5-end region of m-RNA increases the effectiveness of initiation of translation.

b) r-RNAs:
They are class of RNA molecules serving as the component of ribosomes.
Prokaryotic r-RNA:
There are three types of r-RNAs in prokaryotes namely 16S- r-RNA, 23S- r-RNA and 5S- r-RNA.
i) 16S- r-RNA:
This RNA has 1531 nucleotides and is associated with 30S ribosomal subunit of 70S ribosome
ii) 23S - r-RNA:
It has 2904 nucleotides and is associated with 50S ribosomal subunit of 70S ribosome.
iii) 5S- r-RNA:
It has 120 nucleotides and also associated with 50S subunit of 70S ribosome.
16S and 23S r-RNAs are major forms whereas 5S- r-RNA is minor form.
Eukaryotic r-RNA:
There are four types of r-RNAs in eukaryotes namely 18S, 28S, 5.8S and 5S r-RNAs.
i) 18S r-RNA:
This r-RNA has 1900 nucleotides and associated with 40S ribosomal subunit of 80S ribosome. It is synthesized by RNA polymerase I.
ii) 28S, 5.8S and 5S r-RNAs:
These r-RNAs have 4900, 160 and 120 nucleotides respectively. The 18S and 28S r-RNAs are major forms while 5.8S and 5S r-RNAs are minor forms. 28S, 5.8S and 5S r-RNAs are associated with 60S subunit of 80S ribosome. 28S and 5.8S r-RNAs are synthesized by RNA polymerase I but 5S r-RNA is synthesized by RNA polymerase III.
Function:
The precise role of bulk r-RNA in ribosome is not known. However, it is assigned that the major function of r-RNA, is to act as a structural component of ribosome which re complex of r-RNA and protein. Moreover, 3-end of 16S r-RNA has highly conserved sequence complementary to the SD sequence located on m-RNA just prior to an AUG initiation codon. It has sequence of 5 CCUCU 3. Thus, it helps in the binding of m-RNA to ribosome. It has been recently suggested that 5S r-RNA helps in the binding of t-RNA to ribosome because it has a sequence which is complementary to the TYC arm of the RNA.
23S r-RNA found has a role in the peptide bond formation during protein synthesis. 23S r-RNA and six L-proteins are found to have peptidyl transferase activity.
C) t-RNAs (SOLUBLE RNA):
It is a class of RNA molecules each of which combines covalently with a specific aminoacid at the first step in translation in otherwords t-RNAs is carriers of aminoacid to the ribosomes which are sites of protein synthesis. They are made up to 70-90 nucleotides and their molecular weight ranges from 25000 to 30000. The mitochondrial t-RNAs are somewhat smaller in size.
They are small, single stranded molecules which are folded into two-dimensional clover leaf shape. The clover leaf construction of t-RNA consists of three arms with a fourth extra arm occurring frequently. They are
i) DHU arm
ii) TYC arm
iii) Anticodon arm
iv) Extra arm of variable size
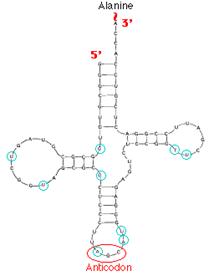
DHU Arm and Loop:
It is called so because of the presence of one or two DihydroUridine residue, which is unusual base at variable position in it. It may also contain 2-o-methyl guanosine residue.
TYC Arm and Loop:
This arm apart from usual bases contains unusual bases like ribothymidine and pseudouridine which is followed by usual base cytidine nucleoside residue. The TYC sequence is a common feature occurring in all t-RNAs. Pseudouridine is structurally similar to Uridine but for C5 of the Uracil base is linked to ribose rather than N-1 as in the case of Uridine.
Anticodon Arm and Loop:
This arm has anticodon corresponding to the triplet codon in m-RNA. The 5-base in Anticodon is the wobble base which has form loose unconventional pairing of triplet codon. The wobble base makes the t-RNA to accommodate more than one codon with the maximum limit of three codons. It also has a conserved purine, uridine and pyrimidine.
5-Terminal:
The 5-end of the t-RNA is usually containing guanosine which is usually phosphorylated.
3-Terminal:
The 3-end of the t-RNA has highly conserved sequence of CCA followed by purine nucleotide. Aminoacids are attached to 2 or 3 carbon of ribose residue of adenosine by aminoacyl t-RNA synthetases (ARSs).
Modified Bases:
There are about 12-15 modified bases found in t-RNA. Of which the following are important one. These modified bases are synthesized by specific t-RNA modifying enzyme. They are: 4-thiouridine, 3-methyl cytidine, 5-methyl cytidine, 7-methyl guanosine, Inosine, N6-methyl adenosine, N6-isopentinyl adenosine, Q-bases ex: Queuosine and Y-Bases ex: Wyosine. Different modifications are introduced at different stages during maturation of t-RNA.
MODIFIED BASE FIGURES
Aminoacyl t-RNA synthetases type-I adds aminoacid to 2-OH of ribose example: Glu, Gln, Arg, Trp etc., Type II ARSs adds aminoacid to 3-OH of ribose of adenosine at 3-end of t-RNA example: Asp, Asn, Ser, Gly etc., t-RNAs when represented along with its corresponding aminoacid indicated at the superscript referred as cognate t-RNA example : t-RNA met cognate t-RNA.
Functions of t-RNAs:
t-RNA molecule carry a specific aminoacid covalently binding to 2 or 3 position of adenine at 3terminal of t-RNAs. Single t-RNA can accommodate a maximum of three codons due to the presence of wobble base. 32 t-RNAs are required for complete protein synthesis. The numbers of t-RNAs are not equal to the number of functional codons i.e. 61. Even though translation possible because single t-RNA can recognize more than one codons. Each aminoacid has atleast one corresponding t-RNA. t-RNAs are highly specific i.e. the t-RNA bind only to one particular aminoacid and not others. TYC arm and DHU arms are responsible for interaction of t-RNA to ribosome.
ii) Ribosomes:
a) Prokaryotic Ribosome 70S ribosome:
The prokaryotic ribosome is made up of two subunits 30S and 50S subunit. Ribosome contains about 65% of RNA and 35% of protein.
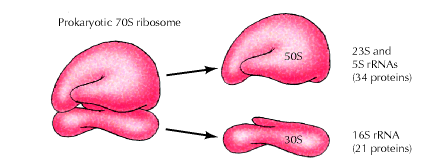
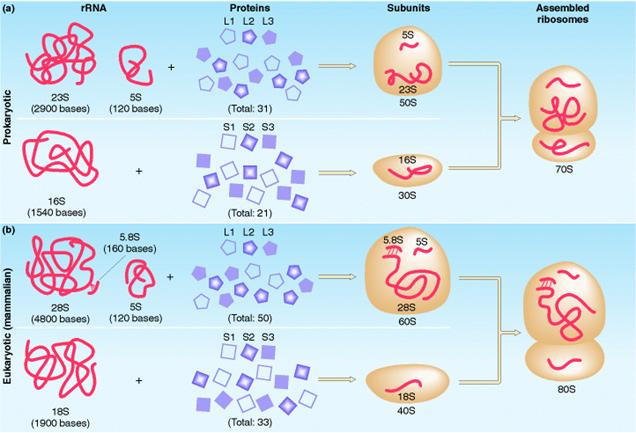
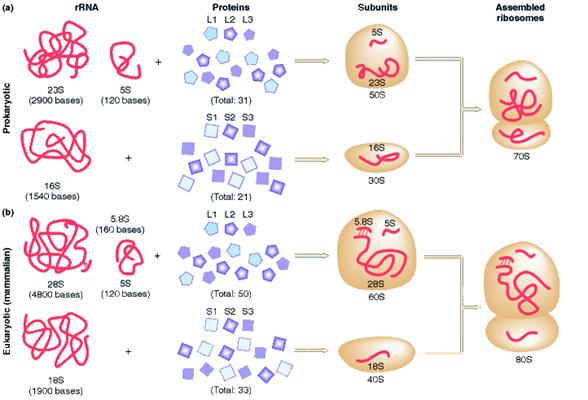
The 50S subunit comprises of 34 proteins (L-Proteins) and 23S and 5S r-RNAs. The 23S r-RNA made up of 2904 nucleotide residues and 5S r-RNA of 120 nucleotide residues. The 30S subunit consists of 21 ribosomal proteins (S-Proteins) and 16S r-RNA molecule which contain 1532 nucleotide residues. Most ribosomal proteins are low molecular weight basic protein. The basic charge reflects their ability to interact with negatively charged RNA. The RNA molecules within the ribosome have defined secondary structure and interact with the ribosomal protein in a defined manner. Prokaryotic ribosome can be dissembled into RNA and protein components and then reassembled into active functional ribosome. Ribosomal proteins are present as single copy except L-7 and L-12 proteins.
b) Eukaryotic Ribosome 80S ribosome:
It has the following structure
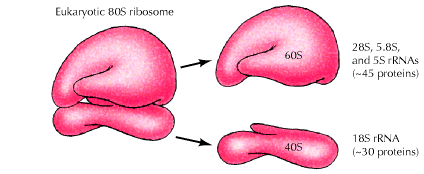
It is made up of two subunits namely large 60S subunit and smaller 40S subunit. 60S subunit contains about 40 to 45 polypeptides and 3r-RNAs components [28S r-RNA, 5.8S r-RNA and 5S r-RNA] and 40S subunit contains about 30 polypeptides and 18S r-RNA components. X-ray diffraction studies have revealed that ribosomes have the following sites
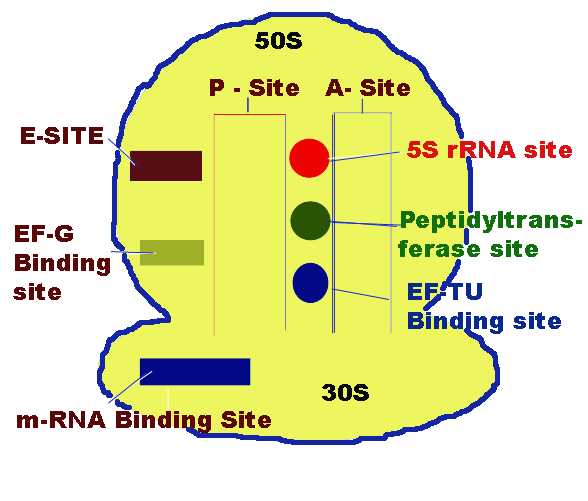
P-Site [Peptide Site]:
It is located on 30S subunit but also extent to 50S subunit. It is the site which binds to initiating t-RNA i.e. N-formyl methionine-t-RNA fmet . During translation peptide containing tRNA present in this site. So, the name Peptide site.
A-Site [Aminoacid Site]:
It lies closely to P-site. It is the site in the ribosome for the binding of incoming aminoacyl tRNA.
m-RNA binding Site:
It is located on 30S subunit. It is associated with 16S r-RNA which carries SD sequence which plays a key role in the m-RNA binding.
Peptidyl transferase site:
It lies somewhere between A and P sites. 23S r-RNA and some of the L-protens are needed for their activity.
5S r-RNA site:
It is located near peptidyl transferase site.
EF-TU binding Site:
It is also located near peptidyl transferase Site. To this site EF-TU binds during elongation of translation. It is present in 50S subunit.
EF-G binding Site:
It is located on the larger subunit of 70S ribosome close to the interface of smaller and larger subunit. To this EF-G binds during elongation of translation.
E-Site:
It is the excision site which is located on 50S subunit. Empty tRNA present at this site during translation before it is freed from ribosome.
iii) TRANSLATIONAL FACTORS:
Different translational factors required to carry out different stages of translation. They are
a) At initiation Stage :- IF1, IF2 and IF3
b) At Elongation Stage :- EF-TU, EF-TS and EF-G
c) At Termination Stage :- RF-1, RF-2 and RF-3
a) Translational initiation (TI) factors:
To begin the translation process, three proteins, acting at different times and sites, are required. These proteins are collectively called initiation factors and are designated IF-1, IF-2 and IF-3. Together these proteins assist in the establishment of an initiation complex and are then released and take no further part in polypeptide synthesis.
IF-1 has Molecular Weight of 9,000. It is required to help IF-3 to separate the 30S subunit from 50S subunit of 70S ribosome and to join an m-RNA at its 5-end, to the freed 30S subunit.
IF-2 has Molecular weight of 25,000. It helps to bind a special charged initiator tRNA and GTP to m-RNA and the 30S subunit. This initiator tRNA recognizes the initiation codon, AUG at the 5-end of m-RNA. Once this complex is formed, a 50S subunit is added to complete a functional ribosome. For each ribosome that attaches to the m-RNA, the same steps are followed.
IF-3 has Molecular Weight of 22,000. It helps in the m-RNA binding and subunit dissociation.
If the m-RNA is polycistronic molecule, IF-2 will assist the complete 70S ribosome to reform an initiation complex at the next initiation codon (AUG) following a stop sequence in the m-RNA. However, the greater the distance between a stop codon and the next initiation codon, the less likely it is that the ribosome will make it to the second or third initiation codon before separating into subunits.
b) Translational Elongation (TE) factors:
In order to bind each successive aminoacid to the polypeptide chain, three or more protein factors are needed. These are called elongation factors and are designated as EF-TU, EF-TS and EF-G.
EF-TU binds charged tRNA and GTP to the ribosome and m-RNA at A-Site. EF-TS release GDP, a GTP hydrolysis product, from EF-TU. EF-TS is also needed to regenerate EF-TU-GTP. Last, EF-G, using GTP as an energy source, promotes movement of the ribosome along the m-RNA as each codon of m-RNA is read and as a new aminoacid is incorporated into the growing polypeptide chain.
c) Translational Releasing Factors (RF):
Set of three proteins, called Releasing Factors (RF) is needed for the separation of the chain from the ribosome once polypeptide synthesis is completed. RF-1 recognizes the stop codons UAA and UAG. RF-2 recognizes stop codons UAA and UGA. RF-3 appears to stimulate the binding of RF-1 and RF-2 to the ribosome in the presence of GTP. The releasing factors acting together or in co-operation with an enzyme peptidyl transferase then stimulate breakage of the bond holding the polypeptide to the last t-RNA, thereby separating the polypeptide from the ribosome.
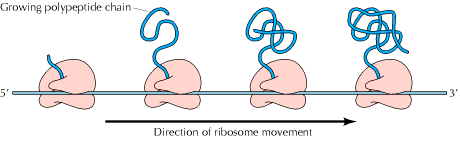
Polysomes (Polyribosomes):
It is a complex of a messenger RNA molecule and two or more ribosomes. When ribosomes are isolated from tissues that very active in protein biosynthesis such as pancreas, they are often assayed in clusters containing several ribosomes. Such clusters of m-RNA and number of ribosomes are called polyribosomes or Polysomes. The formation of polysomes increases the efficiency of translation because the m-RNA is more efficiently used.
GENETIC CODE:
The set of triplet code words in m-RNA, coding for the aminoacid of protein is called genetic code. The triplet code is a codon which is the sequence of three adjacent nucleotide residues in nucleic acid that codes for a specific aminoacid. The code in form of codon is contained in m-RNA which is read by adaptor molecule (t-RNA).
Features of Genetic Code:
Genetic Code found to have the following features:
i) Genetic code is Triplet
ii) It is Non-overlapping
iii) It is Continuous
iv) It is highly Degenerate
v) It has Sense and Nonsense codons
vi) It is Almost Universal
vii) Direction of reading
i) Genetic Code is Triplet:
It is a trinucleotide sequence i.e. it is the sequence of three bases. A single base can specify only four kinds of Aminoacids because only four different types of bases available. If two bases are presumed to be the genetic cods, it can specify only 16 aminoacids [42]. If three, 64 aminoacids can be coded [43]. Since proteins are built from 20 basic aminoacids, it is evident from this simple calculation that three or more bases probably need to specify aminoacids in m-RNA.
ii) Genetic Code is Non-overlapping:
Suppose ABC, DEF, GHI and JKL are set of triplet codes specifying different aminoacids. If the genetic code is Non-overlapping, each triplet should read for only one aminoacid as shown below:
![]()

Non overlapping nature of genetic code has been proved by point mutation experiment. Had the codon being overlapping and if C is mutated then first three aminoacids will be changed because C is expressed in three codons.

But experiments with Tobacco Moasic Virus (TMV) has shown that when one base is mutated then only single aminoacid is get altered. This makes it abundantly clear that codons are Non-overlapping.

iii) Genetic Code is Continuous:
Codons are non-punctuated but are read subsequently from the fixed starting point. This means there are no commas after the reading of each triplet. Had one of the four bases say Q punctuates each triplets as shown below.

Mutation by point deletion or insertion in one of the triplet should not alter the subsequent aminoacid because each triplet is punctuated by Q base. Suppose G base is deleted it has seen that aminoacids aa1 and aa2 remains unaltered but aminoacids aa3 and aa4 get altered because the reading frame shifted leading to deletion.

In the same way, mutation by point addition also leads to altered aminoacid sequence after the point mutation site.

Thus, genetic code studies by point mutation [addition or deletion] have revealed that the codon are continuous without any punctuation and is read in a continuous fashion.
iv) Genetic Code is highly degenerate:
There are 64 possible base triplets and 20S aminoacids. If one triplet code for one aminoacid then 20 triplet codes would have been sufficient. Genetic studies have shown that most of the 64 triplets do code for various aminoacids. This means that some aminoacids are coded by more than one triplet code. The phenomena of having more than one triplet code for the same aminoacid are called degeneracy of genetic code. For example, Aminoacids Ala, Pro, Val, Gly and Thr have four triplet codons. Therefore, they are said to be four fold degenerate while Ser, Leu and Arg have six triplet codons each, therefore said to be six fold degenerates.
v) Functional Nature of Codons:
Out of 64 codons, there are 61 functional or sense codons which specify a particular aminoacids. The remaining three, are terminating or Non-sense codons which are UAG (amber), UAA (ochre) and UGA (opal). The initiation codon is AUG and very rarely GUG.
vi) Genetic code is Almost Universal:
Analysis of mutated genes from plants, mammals, viruses and bacteria has shown that genetic code is almost universal in nature. This means that the aminoacids have mostly the same codons in all living organisms.
vii) Direction of Reading:
The genetic code triplets read in 5ΰ3 direction. Codons starting from initiation codon to until stop codons referred as open reading frame (ORF). It can be considered as single expressable gene.
DECIPHERING THE GENETIC CODE:
It is otherwise called as decoding or breaking the genetic code. Genetic code breaking made possible by M.Grunberg Manago and Ochoa. Because they provided an enzyme Polynucleotide phosphorylase which can synthesize single strand RNA by utilizing nucleoside diphoshpates. This enzyme does not require template and primer.
Genetic code deciphering achieved by the following four experiments namely:
i) Marshell Nirenberg and J.H.Mathei Experiment
ii) H.G. Korana Experiment
iii) M.Nirenberg and Philip Leader Experiment
iv) Invitro Experiment
i) M.W. Nirenberg and J.H.Mathei Experiment (1961):
This experiment was carried out with cell free extracts from bacteria. All the necessary components for protein synthesis except mRNA were present in these extracts. On the addition of chemically defined synthetic mRNAs, the extracts formed specific polypeptides. For example, synthetic m-RNA composed only of U residues yielded polypeptides made up only of phenylalanine. Likewise poly C and poly A coded for single aminoacids Proline and Lysine respectively. Poly G did not work because it assumes unstable stacked structures. Thus three codons are decoded by this experiment.

ii) H.G. Korana Experiment (1968):
When a synthetic m-RNA with alternating A and C residues was added to a protein synthesizing bacterial extract, the resulting polypeptide contained alternating threonine and histidine residues.

A further experiment was needed to determine whether threonine was encoded by ACA and histidine by CAC or vice versa. For this, an m-RNA consisting of repeats of AAC was tested. This m-RNA was found to stimulate the synthesis of three kinds of polypeptide chains : all aspargine, all threonine and all glutamine. This m-RNA can be read in three frames all AAC, all ACA and all CAA. Since only the ACA codon was common to both experiments, it must encode threonine. Thus CAC must encode histidine in the first experiment concluded. Comparisons of the coding capacity of many such mixed polypeptides revealed a substantial part of the genetic code.

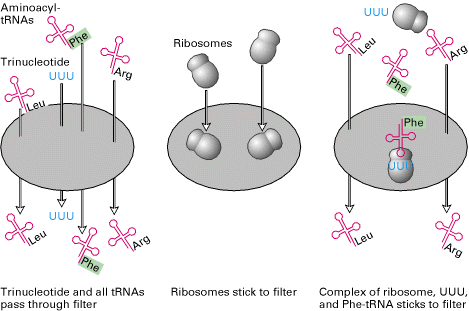
iii) M. Nirenberg and Philip Leader Experiment:
Marshall Nirenberg and his collaborators used extracts of E.Coli to which they added chemically synthesized trinucleotide to decipher the entire genetic code. They prepared 20 bacterial extracts containing all 20 aminoacyl tRNAs. In each extract sample, a different aminoacid was radioactively labeled but the other 19 aminoacids were present on tRNAs remained unlabeled. Aminoacyl tRNAs and trinucleotide passed through a nitrocellulose filter without binding whereas ribosomes did bind to the filter. Each possible trinucleotide was tested separately for its ability to attract specific tRNA by adding it with ribosome to samples from each of the 20 aminoacyl tRNA mixtures. The sample was then filtered.
If the added trinucleotide caused the radiolabeled aminoacyl tRNA to bind to the ribosome, then radioactivity would be detected on the filter. It indicated that the codon codes the corresponding aminoacid. Otherwise, the label would pass through the filter. By synthesizing and testing all possible trinucleotides, the researchers were able to match all 20 aminoacids with one or more codons.
iv) Invitro Experiment:
The first successful synthesis of a specific protein occurred when the m-RNA of bacteriophage F2 was added to bacterial extracts and the coat or capsid protein was formed. With the use of real m-RNAs, it was discovered that AUG encoded Met at the start of almost all proteins and three codons UAA, UAG and UGA did not code any aminoacid but act as terminator or stop codons.
GENETIC CODE DICTIONARY:
After deciphering all codons, genetic code dictionary is formed. It is as follows:
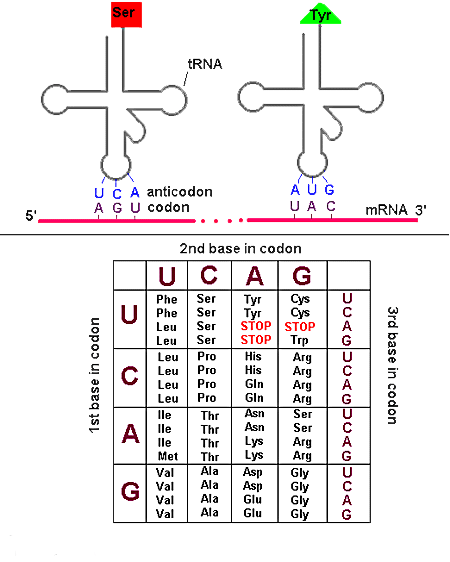
The m-RNA Genetic code. All 64 possible codons are listed with the aminoacids they specify. The codons are read in the 5ΰ3 direction.
DNA codons and the aminoacids they specify i.e. anticoding or template strand codons of DNA is shown in the following figure:
|
|
SECOND POSITION ( middle base) |
Third position |
||||
|
A |
G |
T |
C |
|||
|
F I R S T
P O S I T I O N 3 |
A |
AAA Phe AAG Phe AAT Leu AAC Leu |
AGA Ser AGG Ser AGT Ser AGC Ser |
ATA Tyr ATG Tyr ATT Stop ATC Stop |
ACA Cys ACG Cys ACT Stop ACC Trp |
A G T C |
|
G |
GAA Leu GAG Leu GAT Leu GAC Leu |
GGA Pro GGG Pro GGT Pro GGC Pro |
GTA His GTG His GTT Gln GTC Gln |
GCA Arg GCG Arg GCT Arg GCC Arg |
A G T C |
|
|
T |
TAA Ile TAG Ile TAT Ile TAC Met |
TGA Thr TGG Thr TGT Thr TGC Thr |
TTA Asn TTG Asn TTT Lys TTC Lys |
TCA Ser TCG Ser TCT Arg TCC Arg |
A G T C |
|
|
C |
CAA Val CAG Val CAT Val CAC Val |
CGA Ala CGG Ala CGT Ala CGC Ala |
CTA Asp CTG Asp CTT Glu CTC Glu |
CCA Gly CCG Gly CCT Gly CCC Gly |
A G T C |
|
|
|
5 |
|||||
WOBBLE HYPOTHESIS:
It is a hypothesis given by Crick to explain how one t-RNA molecule can accommodate more than one codons of aminoacids. In order to explain the above anomaly Crick proposed a word wobble which according to him is the relative loose base pairing between base at the 3-end of the codon and the complementary base at the 5-end of the anticodon in the t-RNA. The hypothesis proposes four relationships:
1. The first two bases of a codon always form strong Watson crick basepair with the corresponding bases of anticodon and confer most of the coding specificity.
2. The first base of anticodon [5ΰ3 direction] or the 3rd base [3ΰ5 direction] called the wobble base allows the single t-RNA to adopt more than one codon. The 3rd base [3ΰ5 direction] of the codon leading to loose base pairing which is termed as wobble. The wobble permits t-RNA to read more than one codon with the maximum limits of three codons.
a) If the wobble base is C or A in the anticodon, then it recognize only one codon which most contain G or U respectively in its 3 position. Here C and A form Watson-Crick base pair with G and U respectively.
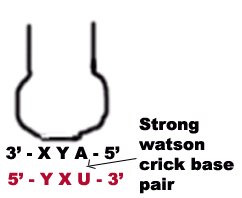
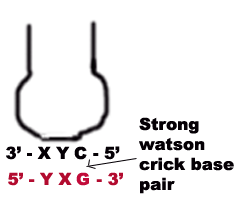
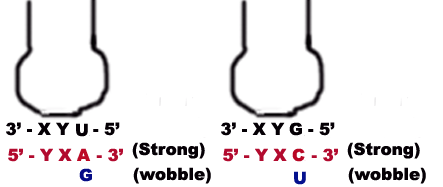
b) When the first base in the 5-end in the anticodon is U then third base in codon can be either A or G. The U forms strong Watson-Crick basepair with A and wobble pairing with G. Similarly if G present at 5 ends of anticodon, then G forms Watson-Crick basepair with C and wobble base pair with U.
c) When I or some other modified base present at 5-end of anticodon then t-RNA can recognize three different codons, all of which form a wobble base pairing at 3 position of codon. The bases that can pair in this case are A, U and C.
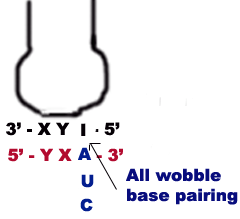
Thus part of degeneracy of the genetic code arises from wobble in the pairing of the third base of the codon.
3. For a given aminoacid and codon that differ in either of first two bases [5ΰ3 direction] requires different t-RNAs.
4. A minimum of 32 t-RNAs are required to translate all 61 different codons for the aminoacids.

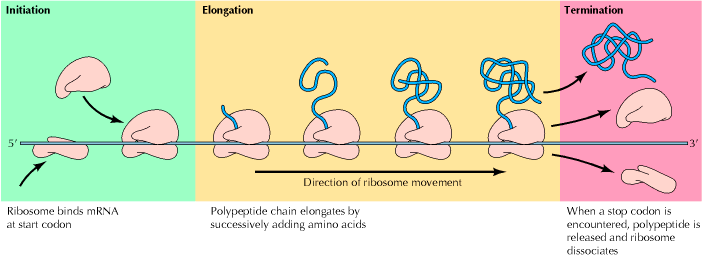
i) Activation of aminoacids
ii) Initiation of polypeptide synthesis
iii) Elongation
iv) Termination
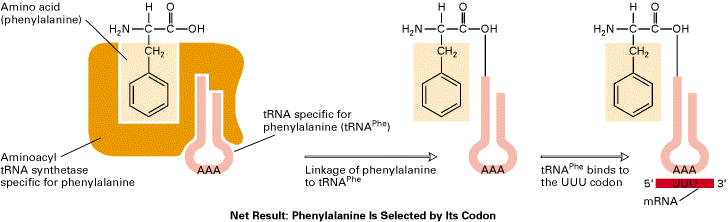
i) Activation of aminoacids:
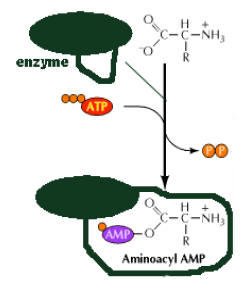
This involves an enzymatically catalyzed covalent binding of an aminoacid to a specific t-RNA at the 3-end at the expense of ATP. The reaction takes place in cytosol in which 20 different aminoacids are esterified to their corresponding t-RNA at their Adenosine residue at 3-end by 20 different activating enzyme called aminoacyl t-RNA synthetases, each of which is specific for one aminoacid and corresponding t-RNA. The overall reaction catalyzed by aminoacyl t-RNA synthetase is given below
The activation occurs in two steps on enzyme catalytic site. In the first step, an enzyme bound intermediate aminoacyl adenylate is formed.
In the second step, aminoacyl group is transferred from enzyme bound aminoacyl adenylate to its corresponding specific t-RNA.
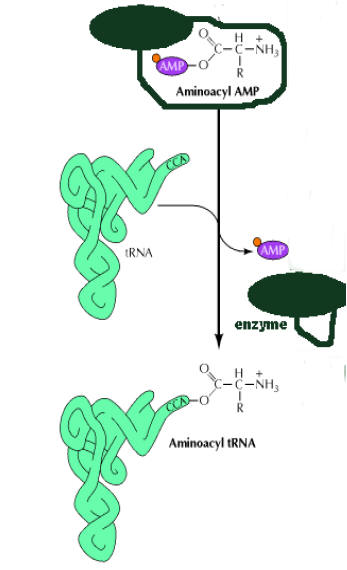
This reaction is irreversible.
Formylation of Methionine:
In E.Coli and other prokaryotes, the starting aminoacid at the amino terminal end is always N-formylmethionine.
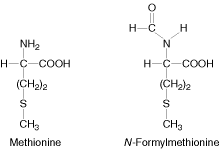
It enters as N-formyl methionyl t-RNAfmet symbolized as metf- t-RNAfmet which is formed in two successive reactions.
a) Methionine is attached to special initiating metf- t-RNAfmet by Methionyl t-RNA synthetase.
b) The formyl group is transferred to amino group of methionine residue from its donor N10-formyl tetrahydrofolate by a specific transformylase enzyme.
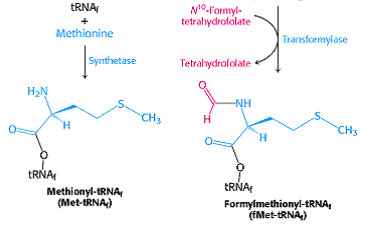
This enzyme cant formylate free methionine. There are two species of t-RNA specific for methionine. One is designated as t-RNAfmet and other t-RNAmet. Both can accept methionine in activation reaction but only t-RNAfmet always can accept formyl group to become the initiating aminoacid.
The other species of methionine t-RNAmet is used to insert methionine in interior position in polypeptide chain. Blocking of the amino group of methionine by N-formyl group not only prevent it from entering into interior position but also allows fmet- t-RNAfmet to be bound at the specific initiation site which does not accept methionine from other aminoacyl t-RNA.
In Eukaryotic cells, on the other hand, all polypeptides are synthesized which begins with methionine residue donated by special initiating methionyl t-RNA (t-RNAimet). Polypeptides synthesized by mitochondria ad chloroplast of eukaryotic cells begins with N-formyl Methionine.ii) Initiation of Polypeptide Synthesis:
The formation of 70S initiation complex constitutes initiation. The conditions required for initiation are
a) m-RNA with initiation codon AUG
b) N-formyl methionyl- t-RNAfmet
c) GTP
d) 30S and 50S subunits of ribosome
e) Mg2+
f) Initiation Factors IF1, IF2, and IF3
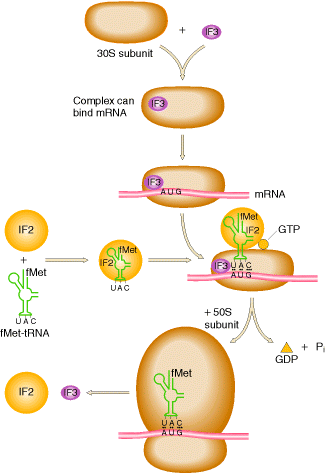
a) Formation of IF3-IF1-30S complex:
Ribosome exists as 70S subunit in the presence of Mg2+ ions. When the Mg2+ concentration decreased, 70S subunit dissociated into 50S and 30S subunit. The initiation factor IF3 binds with the 30S subunit so as to bring about change in shape of 30S subunit which prevents its association with 50S subunit. The other factor IF-1 also binds to smaller subunit. The IF-1 assists the binding of IF-3.
b) Binding of m-RNA:
The binding of m-RNA to 30S subunit takes place such a way that the initiating codon in m-RNA binds to the m-RNA binding site located on 30S subunit. The initiation codon AUG is guided to correct position on 30S subunit by a special signal sequence called shine dalgarno sequence (AGGAGG). Since there is only one codon for methionine which codes for both initiating and interior methionine residues, the initiation signal of SD sequence identify the site where N-formyl met-t-RNAfmet is to be bound. Interior AUG codons are specific for methionyl t-RNAmet and cannot bind N-formyl methionyl t-RNAfmet. This stage results in the formation of IF3-IF1-30S-m-RNA complex.
c) Binding of N-formyl Methionyl- t-RNAfmet:
The IF-3-IF-1-30S-m-RNA complex becomes still larger by binding to IF-2 which already contains GTP and Nfmet t-RNAfmet . During the binding, t-RNA correctly placed on initiating codon.
d) Formation of Initiation Complex:
In this stage, 50S ribosomal subunit combines with large complex with the simultaneous release of initiation factors IF-1, IF-2 and IF-3 which is accompanied by the hydrolysis of GTP to GDP and Pi. This give rise to a functional initiation complex containing N-formylmethionyl t-RNAfmet m-RNA -70S ribosome.
iii) Elongation of Polypeptide Synthesis:
It occurs in three steps namely
a) Binding of aminoacyl t-RNA to A-Site
b) Peptide bond formation
c) Translocation
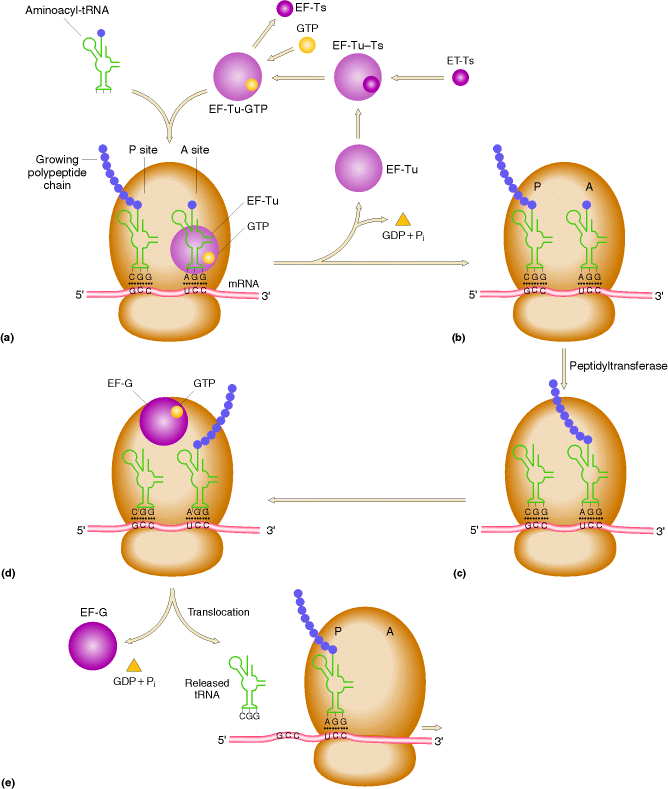
a) Binding of aminoacyl t-RNA to A Site:
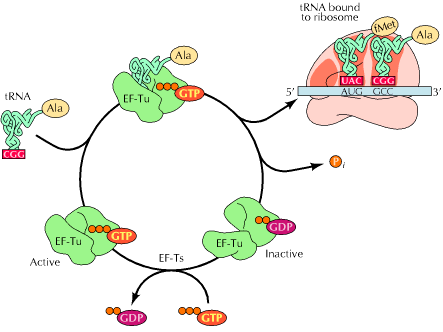
In this step, the aminoacyl t-RNA is attached to the A-site with the help of elongation factors and GTP. First aminoacyl t-RNA binds with a binary complex of EF-TU-GTP to form a ternary complex. It binds to ribosome and during this reaction GTP hydrolyzed into GDP and Pi. After binding, EF-TU-GDP and Pi released. EF-TU-GDP then binds with EF-TS to form EF-TS-EF-TU-GDP complex. GTP then replaces GDP to form EF-TU-GTP along with the release of EF-TS. This EF-TU-GTP complex now ready for the addition of another aminoacyl t-RNA to A site.
b) Peptide bond formation:
In the second step of elongation cycle a new peptide bond is formed between the aminoacids whose t-RNAs are located on A and P sites on the ribosome. This step occurs by the transfer of initiating N-formylmethionine residue from its t-RNA to amino group of the new aminoacid residue that just entered at A site. This step is catalyzed by peptidyl transferase activity provided by 23Sr-RNA and six L-proteins of 50S subunit. 5Sr-RNA and 6 other proteins also aid peptidyl transferase activity. As a result of this reaction a dipeptide if formed on t-RNA at A site and now the empty t-RNA present at P site.
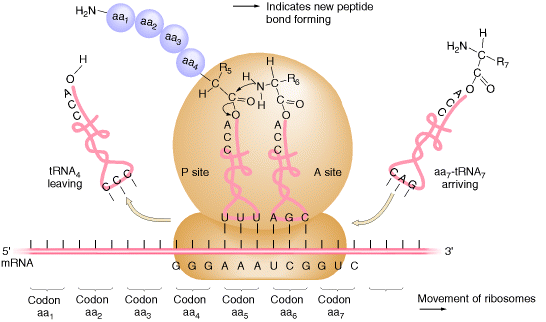
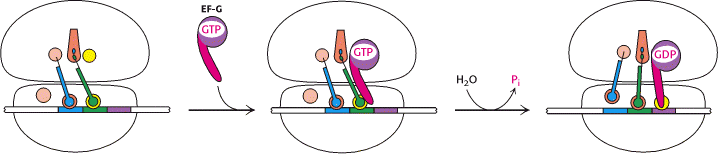
c) Translocation:
In the third step of elongation cycle, the ribosomes move along m-RNA towards its 3-end by the distance of one codon. Since, the dipeptidyl t-RNA is still attached to the second codon of the m-RNA, the movements of the ribosome shifts the dipeptidyl t-RNA from the A site to P site which causes the release of preceding t-RNAfmet which is emptied from the P site. This shift of ribosome along m-RNA is called translocation. This requires elongation factor EF-G which is also called as Translocase. The hydrolysis of GTP provides energy for Translocation.
The ribosome with its attached dipeptidyl t-RNA and m-RNA is now ready for another elongation cycle to attach the third aminoacid residue which proceeds in precisely the same way as the addition of second.
iv) Termination:
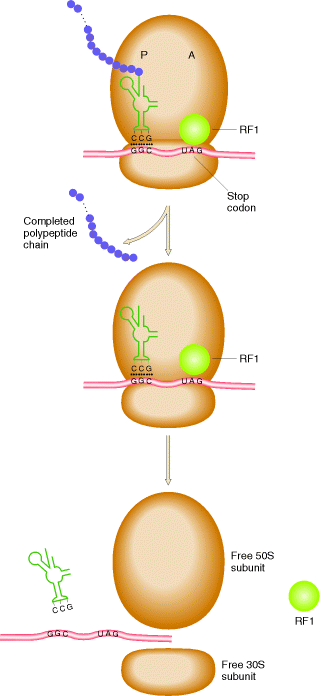
The polypeptide chain continues to grow until one of the stop codons [UAA, UAG or UGA] is reached. These codons do not specify any aminoacid and have no t-RNA to pair with them. When these codons are reached, they are recognized by release factors which are one of these types RF1, RF2 and RF3. Release factors recognize four nucleotides in m-RNA along with the hydrolysis of GTP. RF1 recognizes UAA and UAG. RF2 recognizes UAA and UGA. RF3 binds with GTP, and stimulate the binding of RF1 and RF2 to ribosomes. All these factors act at the ribosome A site. But recently identified that UAG and UGA codes for Pyrrolysine and selenocystein respectively.
EUKARYOTIC TRANSLATION:
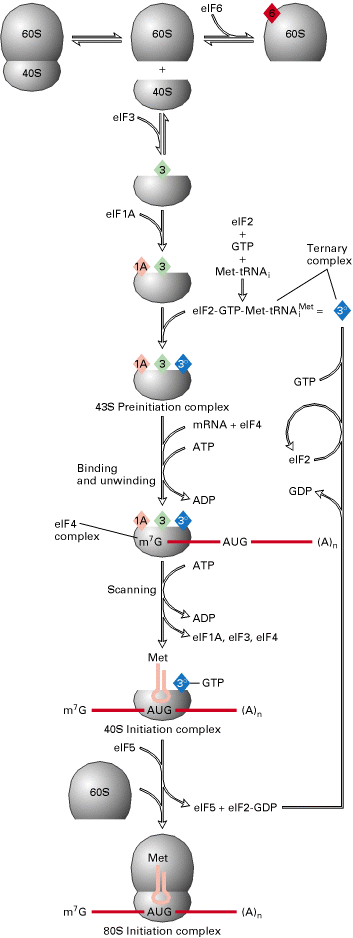
Eukaryotic translation occurs in four stages namely:
I) Activation of Aminoacids
II) Initiation
III) Elongation
IV) Termination
I) Activation of Aminoacids:
As in prokaryotes, aminoacids are activated by binding to t-RNA. The initiating aminoacyl t-RNA is met-t-RNAimet instead of met-t-RNAifmet.
II) Initiation:
It occurs in five steps. In first step, eIF2, GTP and met-t-RNAimet bind to form ternary complex. In the second step, ternary complex bind to 40S-eIF1-eIF3-eIF4C complex to form 40S-t-RNA complex. In the third step, m-RNA-4f-4a-4b complex bound to the above complex along with the release of eIF4a, 4b and 4f. This reaction is derived by ATP hydrolysis.
In the fourth step, t-RNA recognizes initiation codon and bound to it. In the final step, 60S subunit of 80S ribosome bound along with the release of eIF1, 3, 4C and 2. eIF-5 catalyzes this step. Hydrolysis of GTP occurs in this step. Initiation complex 80S-m-RNA- met-t-RNAimet formed.
III) Elongation:
Elongation occurs in three steps as in prokaryotes namely
a) Binding of aminoacyl t-RNA to A site
b) Peptide bond formation
c) Translocation
The main difference between prokaryotes and eukaryotes is that the elongation factors. In eukaryotes the elongation factors are eEF1a, eEF1bg and eEF2.IV) Termination:
A Single factor eRF-1 or eTF-1, was found to catalyze the release of the completed polypeptide chain from eukaryotic ribosomes. This appears to recognize all three termination codons UAA, UAG and UGA. GTP hydrolysis is required for termination.
INHIBITORS: PROKARYOTIC INHIBITORS:
|
S.No |
Name |
Mechanism of Action |
|
1. |
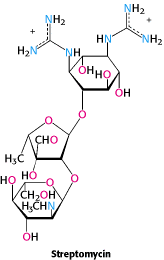
Streptomycin, Neomycin and Kanamycin |
Binds to 30S subunit to cause misreading and inhibition of initiation. |
|
2. |
Paromomycin |
Inhibit initiation. Resistant mitochondria have altered small r-RNA. |
|
3. |
Tetracycline |
Inhibits elongation by blocking binding of aminoacyl t-RNA to the A site on the 30S subunit. |
|
4. |
Chloramphenicol |
Inhibits elongation at Peptidyl transferease activity. |
|
5. |
Erythromycin |
Inhibits elongation at Transpeptidation step. |
|
6. |
Spectinoycin |
Inhibits elongation at transpeptidation. Resistant ribosomes have altered potein S5. |
|
7. |
Thiostrepton |
Inhibits elongation, preventing binding of EF-G-GTP complex to ribosome. |
|
8. |
Kirromycin |
Inhibits elongation, preventing release of EF-TU-GDP complex from ribosome. |
|
9. |
Colicin E3 |
Specific nuclease for site on 16S r-RNA. |
|
10 |
Trimethoprin |
Prevents formation of fmet-t-RNA by inhibition of synthesis of N10-formyl THF. |
|
11. |
Linomycin |
Inhibit peptidyl transferase complex |
|
12. |
Kasugamycin |
Inhibit binding of aminoacylt-RNAfmet . |
EUKARYOTIC INHIBITORS:
|
S.No |
Name |
Mechanism of Action |
|
1. |
Cycloheximide (actidione) |
Inhibits elongation and initiation, freezing ribosomes on polysomes (peptidyl transferase). |
|
2. |
Emetine |
Inhibits elongation at translocation step. Resistant hamster cells have altered protein S14. |
|
3. |
Diphtheria toxin |
Inhibits elongation by inactivating EF-2 by ADP ribosylation. |
|
4. |
Ricin and abrin |
Inhibits elongation, affecting 60S subunit. |
INHIBITORS OF BOTH:
|
S.No |
Name |
Mechanism of Action |
|
1. |
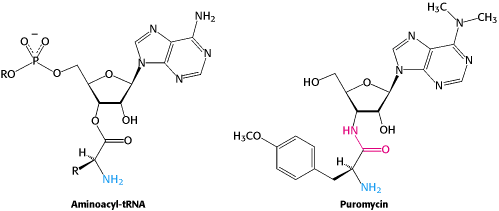
Puromycin |
Inhibits elongation due to premature termination by acting as analogue of charged t-RNA. |
|
2. |
Pactamycin |
Inhibits initiation. It was used to determine gene order of proteins derived from picornaviral polyproteins. |
|
3. |
Aurintricarboxylic acid |
Inhibits initiation by preventing binding of m-RNA to ribosome. |
|
4. |
Showdomycin |
Inhibits initiation at the stage of ternary complex formation [IF2-t-RNA-GTP]. |
|
5. |
Sparsomycin |
Inhibits elongation at Peptidyl transferase step. |
|
6. |
Fusidic acid |
Inhibits elongation, preventing release of EF-G-GDP complex from ribosome. Resistant bacteria have altered EF-G. |
|
7. |
a-Sarcin |
Specific nuclease for site on 23S and 28S r-RNA in ribosomes although inactive against intact E.Coli. |
|
8. |
5-flurotryptophan |
Prevents activation of t-RNAtrp by competitive inhibition of synthetase. |
|
9. |
Norvaline |
Prevents activation of t-RNAval. |
|
10. |
Ethionine |
Causes synthesis of abnormal proteins with ehionine instead of methionine. |
|
11. |
O-Methylthreonine |
Causes synthesis of abnormal proteins with O-methylthreonine replacing threonine. |
FIGURES FOR PROTEIN INHIBITORS
REGULATION OF TRANSLATION:
PROKARYOTIC TRANSLATIONAL CONTROL:
In prokaryotes, translational control is of lesser importance than transcriptional control for two reasons. First, messenger RNAs are extremely unstable, so there is no need to control rate of translation because m-RNA lysed immediately. Second, although there are some indications of translational control in prokaryotes, such control is inefficient because energy is wasted in synthesizing m-RNAs that may never be used. Different ways in which prokaryotic translation controlled are as follows:
a) Gene location
b) Antisense RNA
c) Efficiency of m-RNA to bind with ribosome
d) Codon preference
e) Stringent Response
f) Attenuation
a) Gene Location:
Translation rate ratio of genes in Lac operon from 5-end, is 10:5:2. The reason for this is that in prokaryotes transcription and translation occur hand by hand so, genes at the beginning of operon [5-end] translated well before, the genes at the 3-end. In addition to this, exonuclease seems to degrade m-RNA more efficiently from the 3-end. Thus, genes located at 5-end of operon found to have higher translation rate than at 3-end. Usually, biologically active genes present at 5-end.
b) Antisense RNA:
Translation can also be regulated by RNA-RNA hybridization. RNA complementary to the 5-end of a m-RNA can prevent the translation of that messenger RNA. Several examples of this type of regulation are known. The regulating RNA is called antisense RNA. It is synthesized a direction which is opposite to the direction of m-RNA. Example: The m-RNA from the omp-F gene in E.Coli is prevented from being translated by complementary base pair binding with an antisense RNA called mic F-RNA.
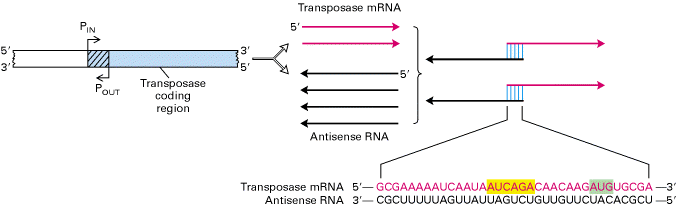
c) Efficiency of m-RNA to bind with ribosome:
A third translational control mechanism consists of the efficiency with which the m-RNA is bound to the ribosome. This efficiency is related to some extent to the sequence of nucleotides at the 5-end of the messenger RNA that is complementary to the 3end of the 16S r-RNA in ribosome i.e. Shine Dalgarno sequence. Variations from this consensus sequences have different efficiencies of binding and therefore, the initiation of translation occurs at different rates.
d) Codon Preference:
Even though degeneracy present for aminoacids, in different species, specific codons are preferred to code for particular aminoacids. This is referred as codon preference. Thus, genes with preferable codons expressed at higher rate whereas ones with other codons expressed at slower rate.
e) Stringent Response [Idling reaction]:
INCLUDE NOTES FROM TRANSCRIPTIONAL REGULATION
f) Attenuation:
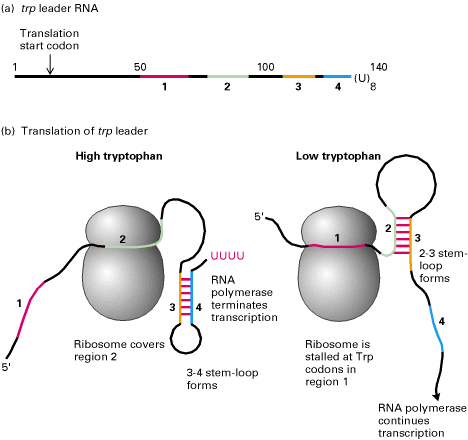
INCLUDE NOTES FROM TRANSCRIPTIONAL REGULATION
EUKARYOTIC TRANSLATION CONTROL:
Eukaryotic m-RNAs are much longer lived than prokaryotic ones, so there is more opportunity for translational control. The rate limiting step in translation is usually initiation, so mostly control exerted at this level. Regulation achieved mainly in two ways. They are:
i) phosphorylation
ii) Interaction by RNA binding Protein
i) Phosphorylation:
Phosphorylation of some of the regulatory factors inhibit translation whereas in some other cases it ca be stimulatory.
a) Inhibitory phosphorylation:
The best known example of inhibitory phosphorylation occurs in reticulocytes where in the absence of Heme, Heme controlled repressor (HCR) or Heme regulated inhibitor (HRI) phosphorylates one of the subunits of eIF2, known as eIF2a . The phosphorylated form of eIF2 binds more tightly than usual to eIF2B, which is an initiation factor whose job it is to exchange GTP for GDP on eIF2. When eIF2B is stuck fast to phosphorylated eIF2; it cannot get free to exchange GTP for GDP on other molecules of eIF2, so eIF2 remains in the inactive GDP-bound form and cannot attach met-tRNAimet to 40S ribosomes. Thus, translation initiation inhibited.
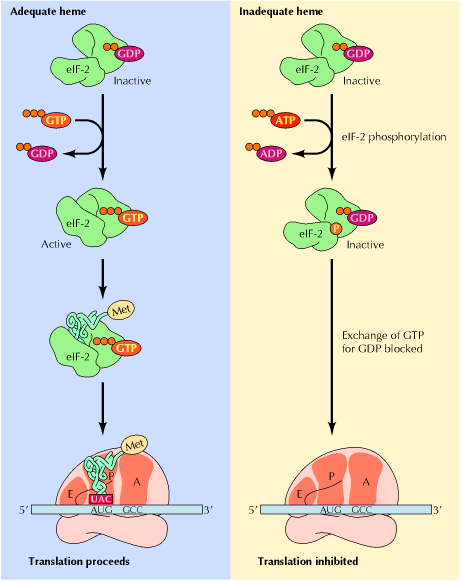
b) Stimulatory phosphorylation:
Insulin or growth factors such as EGF bind to its receptors at the cell surface. Through a series of steps these activates the signal transducer Ras, which through another series of steps, activates MAP kinase. This kinase has many targets, one of which is PHAS-1. When PHAS-1 is phosphorylated by MAP kinase, it dissociates from cap binding protein eIF4f. Now eIF4f, free to participate in translation. Thus phosphorylation stimulates translation.
ii) Interaction with RNA binding Protein:
RNA binding proteins by its interaction with RNA, it may stimulate or inhibit translation. Examples for this are as follows:
a) Stimulation of Translation:
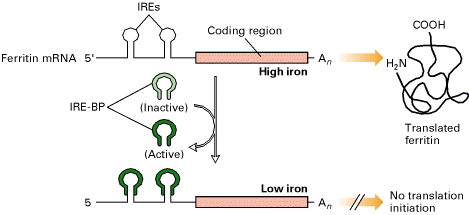
The 3-Iron Response Element (IREs) in the transferring receptor (TfR) m-RNA have a stem loop structure containing AU rich sequence that promotes m-RNA degradation at high intracellular iron concentrations. At low intracellular iron concentrations, the concentration of IRE-BP is such that it binds to the IREs, thereby inhibiting degradation. As a result, the translation of TfR m-RNA increased because of IRE-BP.
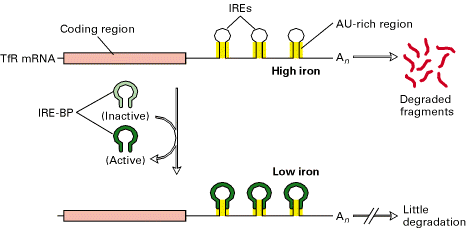
b) Inhibition of Translation:
Ferritin m-RNA contain IRE at 5-end. At low iron concentrations, IRE-BP binds to IREs and inhibit translation of ferritin mRNA. The same mechanism controls translation of the mRNA encoding ALA synthetase. In these two m-RNAs, the IREs do not contain the AU rich sequences that promote m-RNA degradation as they do in transferring receptor mRNA.
POST TRANSLATIONAL MODIFICATIONS:
The newly synthesized polypeptide chain by the ribosome often doesnt attain its final biologically active conformation until it has been subjected to processing of covalent modification of polypeptide chain or protein after the biosynthesis, so that they are rendered biologically active, is called post translational processing or post ribosomal modification. Several kinds of processing may takes place depending upon the nature of protein and they are as follows:
I. Covalent modification
II. Non-covalent modification
I. Covalent modification:
1. Covalent modification by charge removal:
This is done by amino terminal and carboxy terminal modification in which the charge of the amino acid and carboxy terminal of the protein are removed. The amino terminal is modified by acetylation.

The carboxy terminal is modified by converting to amides.

Gastrins (hormones) are complexes of polypeptide which modified by the acetylation of their amino terminal amino group. Modification of terminal carboxy group by terminal amidation takes place in several small peptide hormones. For example, thyrotropin releasing factor in which the carboxy terminal of proline of tripeptide is amidated.
2. Covalent modification by adding negative charges:
This is done by mainly by phosphorylation which is reversible and one of the most common post translational modifications. In phosphorylation the amino acids which are most commonly phosphorylated are serine, threonine and tyrosine, lysine, arginine, and histidine group of protein are also phosphorylated, but are less common. These aminoacids are enzymatically phosphorylated by ATP.
Significance of post translational modification by phosphorylation:
There are many regulatory enzymes which plays crucial roles in the metabolism of that are covalently modified by phosphorylation. This being a reversible modification helps in the interconversion of enzyme from active to inactive form. Important example is the enzyme glycogen phosphorylase of muscle and liver which catalyzes the following reaction
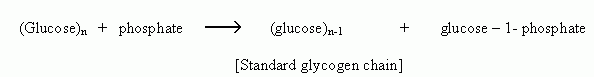
The enzyme glycogen phosphorylase exists in two forms: glycogen phosphorylase b relatively active form and glycogen phosphorylase a more active form.
The inactive phosphorylase b is converted to active phosphorylase a by an enzyme phosphorylase kinase which phosphorylates the OH group of the serine. The phosphorylation of serine residue makes the enzyme active because they happened to be situated in their active centre i.e. catalytic site.
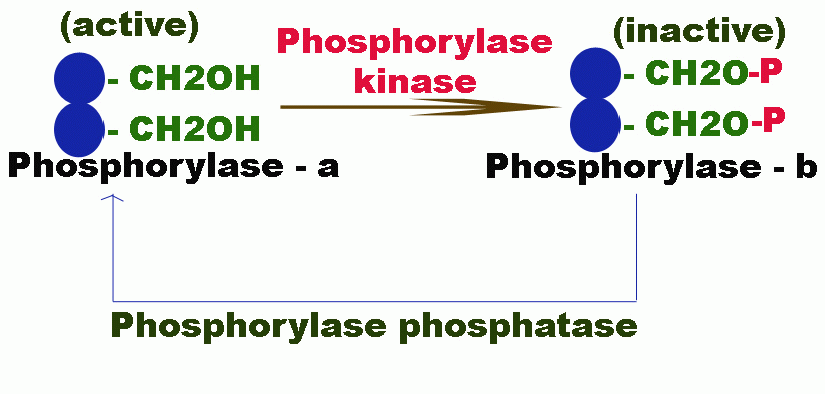
The active form of the enzyme is again converted back to its inactive form b by another enzyme phosphorylase phosphatase.
Phosphorylation of some protein controls the cell cycle. Phosphorylation also modulates the activity of many peptide hormones. Milk protein casein is also phosphorylated to its serine residue due to which it gets bound to calcium ions. They help in providing calcium as a nutrient phosphorylation of specific tyrosine residue has been found to be an important step in the transformation of normal cells into cancer cells.
3. Covalent modification by ADP ribosylation:
Some proteins get modified by adding ribose to carboxylic group in proteins. The ADP-ribose is donated by NAD+. For example, the eEF2 gets modified by addition of ribose to the carboxy group of histidine, when infected with Cornebacterium diptheriae.
4. Covalent modification by methylation:
Several proteins are modified by methylation some protein lysine residues are methylated enzymematically. For example: In some muscle cell protein and cytochrome, the lysine residue and structural function. Lysine residue of some nucleoproteins may have 1-3 methyl groups.
Methylation of aspartate residue occurs in membrane protein. Methylation of either glutamate or aspartate residue destroys the negative charge. The methyl esters are readily hydrolyzed to regenerate the original carboxy groups. So, methylation of this type can be used as reversible control devices. In some of muscle protein histidine residue are methylated.
In some proteins the arginine residue are methylated especially in cell nuclei, nerve sheaths and sperm tails, sperm immobility, a common cause of human infertility is often caused by defective metabolism of sperm protein.
5. Covalent modification of hydroxylation:
In protein collagen, proline residues are hydroxylated to hydroxyproline in endoplasmic reticulum, reaction that increases the strength of collagen.
6. Covalent modification by carboxylation:
In some protein an extra carboxyl group is added to aspartic acid and glutamic acid residues. For example in blood clotting protein prothrombin a number of glutamic acid residues are carboxylated to form carboxy glutamate which increases its affinity for calcium ions. Thus it helps in initiating blood clotting.
7. Covalent Modification by glycosylation:
Many proteins that function excellulary, secretary protein ment for extracellular transport are glycosylated. For example, some of the digestive enzyme peptide hormones, membrane proteins, antibodies are glycosylated. The carbohydrate is linked mostly to aspargine and more rarely to serine or threonine residue.
Modification of glycosylation occurs both in endoplasmic reticulum and Golgi apparatus. Glycosylated involves addition of oligosaccharide consisting of N-acetyl glucosamine, mannose and galactose. Further fucose and N-acetyl neuraminic acid (NANA) are added in Golgi apparatus. Glycosylation helps in targeting and protection of proteins. For example thyroglobulin secreted by thyroid cells is glycosylated. Many proteins that lubricate mucous membrane are also glycosylated.
8. Covalent modification by addition of prosthetic group:
Many enzymes contain covalently bound prosthetic group necessity for this activity. These are attached to the polypeptide chain after it cleaves the ribosome. For example biotin molecules [prosthetic group] are covalently bound to acetyl CoA carboxylase and heam group to cytochrome C.
II) Non-covalent modification:
They include following:
1. Partial hydrolysis:
Enzymes involved in digestion and proteins involved clotting and some peptide hormones are modified by this method. Digestive enzymes are usually produced in their native form called zymogen because they may prove to be fatal; If they are in their active form within the cells therefore enzyme reaching the digestive track under set conditions gets converted into their active form. This modification involves partial hydrolysis of extra polypeptide chain. For example, pepsinogen, prothrombin, proinsulin get converted to their active form by partial hydrolysis.
2. Disulphide crosslink formation:
Many proteins after their biosynthesis form disulphide linkage so that they may attain a conformation to be physiologically active. For example insulin and ribonuclease enzyme require disulphide bridge formation for their catalytic activity. The disulphide bridges are formed between cysteine and methionine residue.
3. Addition of signal sequence and their removal:
Some newly made protein will simply be delivered into the cell cytosol. Some will deliver to different cell organelles. Some will be secreted into the exterior of the cell and some will be inserted into the cell membrane. It is therefore important that synthesized protein find its way to its correct site in the cell. This is done by the addition of signal sequence to the protein.
Many proteins contain specific polypeptide leaders on their amino terminal end that function as signals spot direction then to the proper destination. They are called signaling sequence, which have 15-30 aminoacid residues many of which are hydrophobic R-groups. The proteins which have to be exported out have to be targeted to Golgi bodies from where they are secreted out. Thereafter signal sequence is removed from the protein by the introns of special peptides.
The protein which are meant for organelle such mitochondria and after reaching the organelles, the signal sequence hence help in the transport of massive protein across the organelle membrane.