ANTIBODY PRODUCING CELL (B cell):
Antibody producing cell is B lymphocyte. But B lymphocyte has different names like Pre-B cell, Immature B cell, mature B cell, Naive B cell, effector B cell, Plasma cell and memory B cell. These names provided to B cells at different period of their life time and place of occurrence.

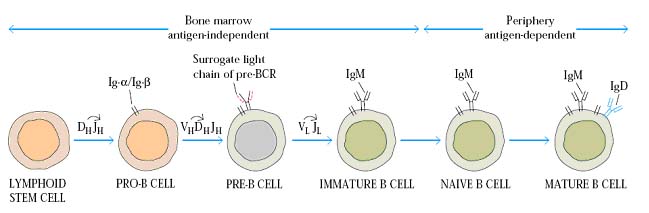
Normally B cells are produced in Bone marrow from pluripotent stem cells of bone marrow. Once the stem cell committed to develop into B cell it is called as Precursor B cell or Pre-B cell. Pre-B cell does not posses B cell receptor. During B cell development an important process known as DNA rearrangement occurs for Ig genes, at this stage Pre-B cell is called as immature B cell. After rearrangement completed, genes start to express and B Cell Receptor (BCR) appears on the membrane of immature B cell because BCR is mainly of immunoglobins. At this stage immature B cell said to be mature B cell. They posses well developed BCR which is made up of membrane bound Ig M and Ig D. Thus B cell development and maturation of B cell occurs in primary lymphoid organ Bone marrow. These mature B cells then start to move towards secondary or peripheral lymphoid organs to encounter antigen. During their transport, they are known as naive B cells i.e. they are not exposed to antigen.
In secondary or peripheral lymphoid organ, naive B cell encounters antigens. Antigen is recognized by B cell through BCR and its co-receptor. Antigen recognition can activate B cell in a T-cell dependent or independent manner depending upon the nature of antigen. After activation, B cell undergoes proliferation. After proliferation, with the help of cytokines, they undergo differentiation process through which two types of cells produced namely effector B cell (Plasma cell) and memory B cell. Plasma cell is the type of B cell which can secrete antibodies depending upon the cytokines and class switching process. All plasma cell initially produce Ig M type and latter they switched to produce either Ig G or Ig A or Ig E. They are also actively involved in primary immune response i.e. present infection clearance reaction. Memory B cell does not produce secretary antibodies but they have membrane bound immunoglobins which is used for antigen recognition in the future. They place vital role in secondary immune response. Once B cell differentiated to effector B cell and memory B cell, they can move to tertiary lymphoid organ for their action.
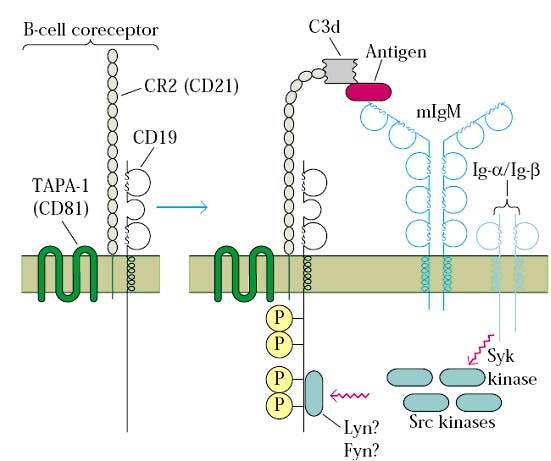
B cell receptor and co-receptor:
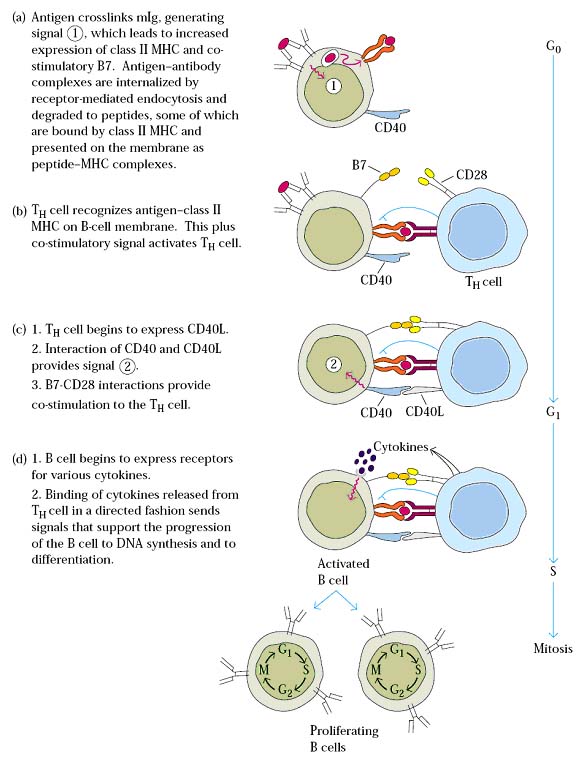
B cell activation and signaling by T-cell dependent antigen:
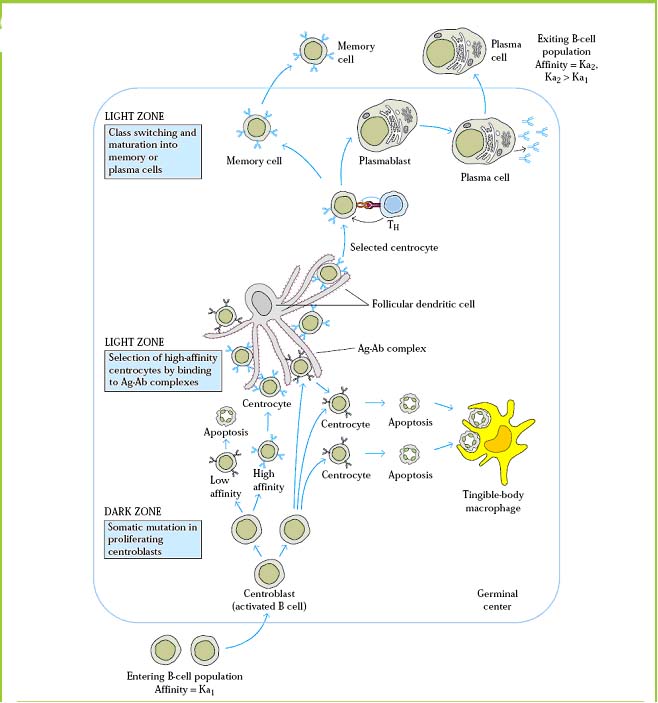
Proliferation, Selection and differentiation:
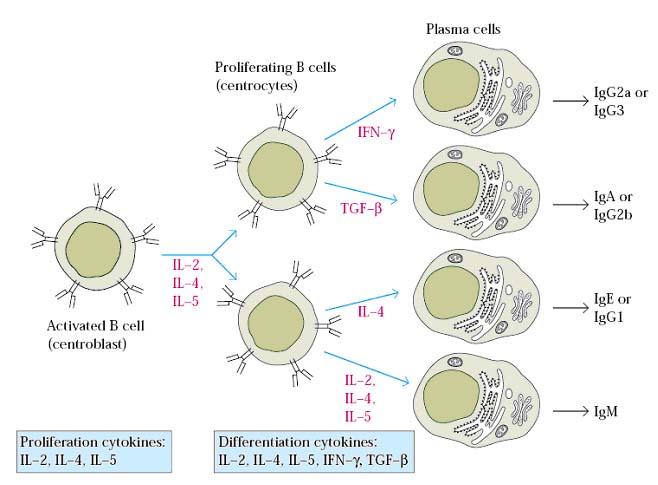
B-cell differentiation by cytokines:
THEORIES FOR ANTIBODY PRODUCTION:
There are five theories are proposed for antibody production. They are as follows:
1. Side chain theory
2. Direct template theory
3. Indirect template theory
4. Natural selection theory
5. Clonal selection theory
1. Side chain theory:
It is proposed by Ehrlich in 1897. According to this theory, B cell contains different side chains i.e. antibodies with different specificity. When antigen encounters naive B cell, it can interact with the side chains and select one of the side chains. It intern activates B cell. Activated B cell then produces antibodies of selected side chain type.
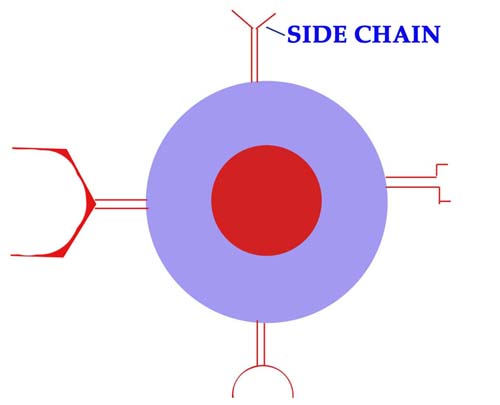
But in reality, each B cell possesses only one type of BCR with single specificity. Because of this, this theory disproved.
2. Direct template theory:
It is proposed by Breinl and Haurowitz in 1930. According to this theory, antigen enters into B cell and act as template for the production of antibody. Since this antibody is produced from antigen, it is specific for antigen. But in reality, the antibody specificity is predetermined before encountering antigen because maturation of B cell occurs in antigen independent phase in bone marrow. Because of this reason, direct template theory also disproved.
3. Indirect template theory:
Burnet and Fenner proposed this instructive theory to explain the synthesis of antibody as an adaptive protein. According to this theory, antigen enters into B cell and it binds to its DNA and modifies it and forms this modified DNA, antibodies are produced against antigen and it also specific for it. But in nature, there is no need for an antigen to enter into B cell and modify DNA for antibody production because soluble antigen can activate B cell by binding to its cell surface receptor BCR. Because of this reason, this theory also disproved.
4. Natural selection theory:
This theory is proposed by Jerne in 1955. According to this theory serum will contain antibodies for all antigens and these antibodies are known as natural antibodies. Antibodies for blood group antigen A and B can be given as an example for this theory. But naturally serum will not contain secreted antibodies for specific antigen till it enters host and activates B cell. Because of this valuable reason, this theory also rejected.
5. Clonal Selection theory:
This is the present day theory which is also accepted one. This theory was proposed by Burnet in 1957.
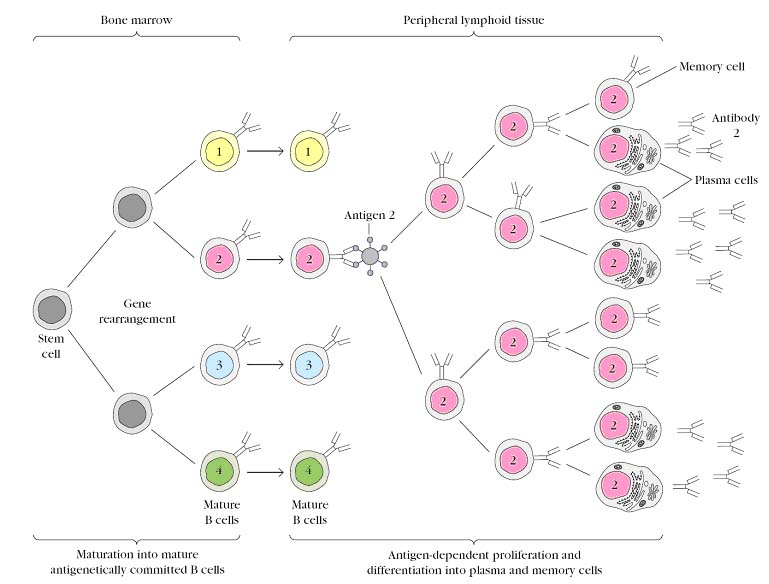
According this theory, the antigen specificity is predetermined one i.e. B cell is matured with single antigen specificity in bone marrow and B cells with same specificity is referred as B cell clone for a specific specificity. After they matured, they moved to peripheral lymphoid organs. When antigen enters, it moved to peripheral lymphoid organs for activation. In this process, antigen selects a single clone from multiple B cell clone with different specificity. Thus this theory named as clonal selection theory. After a clone is selected by antigen, B cell is activated and proliferated. After proliferation, selected B cells undergo differentiation to form plasma cell and memory B cells for specific antigen. Plasma cells secrete antibodies and they participate in antigen removal and memory cells wait for future antigen encounters. This theory is also practically proved.
ANTIBODY PRODUCTION:
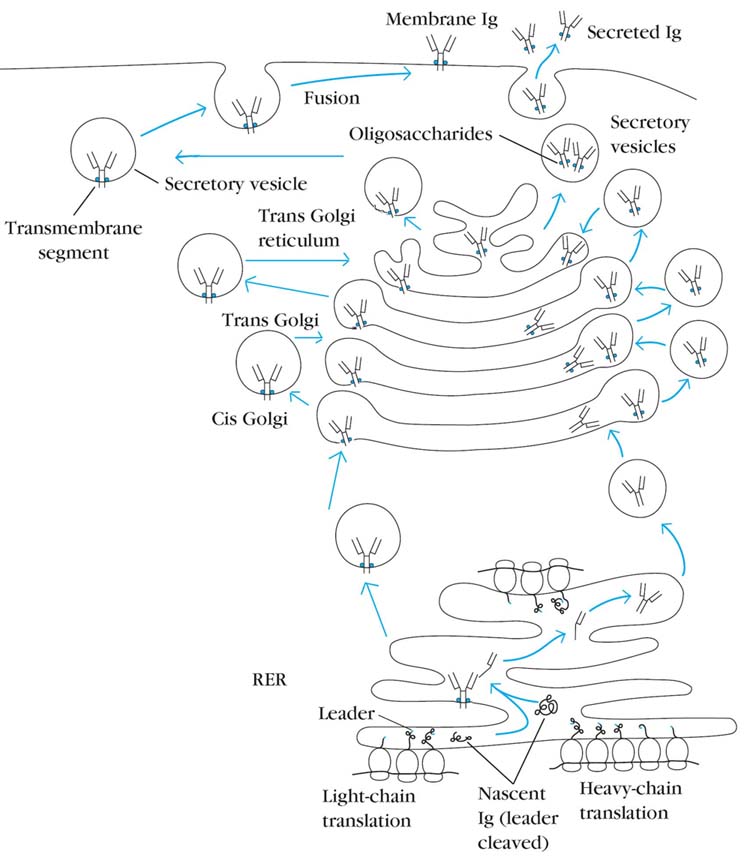
Immunoglobulin heavy- and light-chain mRNAs are translated on separate polyribosomes of the rough endoplasmic reticulum (RER). Newly synthesized chains contain an amino-terminal leader sequence, which serves to guide the chains into the lumen of the RER, where the signal sequence is then cleaved. The assembly of light (L) and heavy (H) chains into the disulfide-linked and glycosylated immunoglobulin molecule occurs as the chains pass through the cisternae of the RER. The complete molecules are transported to the Golgi apparatus and then into secretory vesicles, which fuse with the plasma membrane and antibody either present on membrane (BCR) or secreted out. The order of chain assembly varies among the immunoglobulin classes. In the case of IgM, the H and L chains assemble within the RER to form half-molecules, and then two half-molecules assemble to form the complete molecule. In the case of IgG, two H chains assemble, then an H2L intermediate is assembled, and finally the complete H2L2 molecule is formed. Interchain disulfide bonds are formed, and the polypeptides are glycosylated as they move through the Golgi apparatus. If the molecule contains the transmembrane sequence of the membrane form, it becomes anchored in the membrane of a secretory vesicle and is inserted into the plasma membrane as the vesicle fuses with the plasma membrane. If the molecule contains the hydrophilic sequence of secreted immunoglobulins, it is transported as a free molecule in a secretory vesicle and is released from the cell when the vesicle fuses with the plasma membrane.
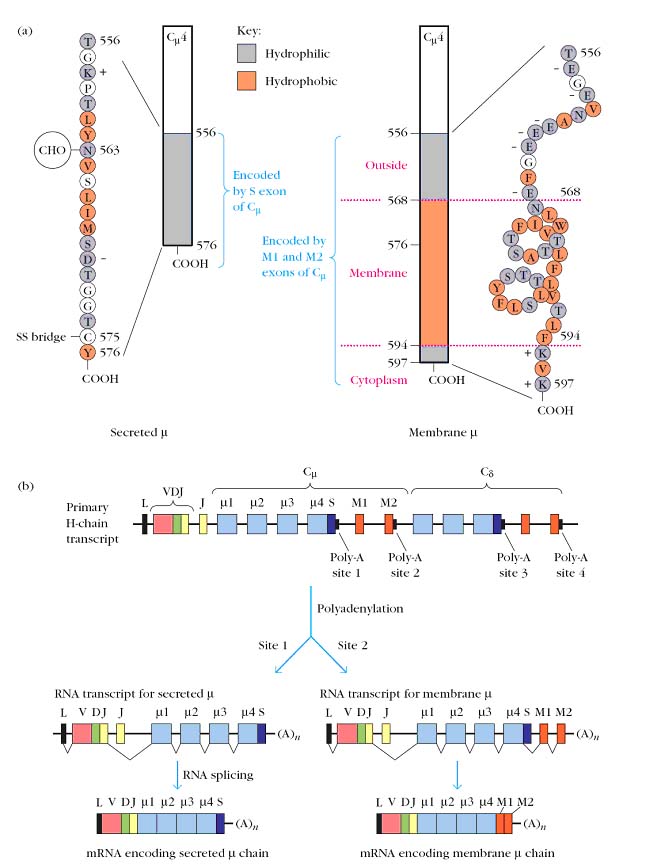
A particular immunoglobulin can exist in either membrane-bound or secreted form. The two forms differ in the amino acid sequence of the heavy-chain carboxyl-terminal domains (CH3/CH3 in IgA, IgD, and IgG and CH4/CH4 in IgE and IgM). The secreted form has a hydrophilic sequence of about 20 amino acids in the carboxyl terminal domain; this is replaced in the membrane-bound form with a sequence of about 40 amino acids containing a hydrophilic segment that extends outside the cell, a hydrophobic transmembrane segment, and a short hydrophilic segment at the carboxyl terminus that extends into the cytoplasm. The primary transcript produced by transcription of a rearranged _ heavy-chain gene contains two polyadenylation signal sequences, or poly-A sites, in the C_ segment. Site 1 is located at the 3_ end of the C_4 exon, and site 2 is at the 3_end of the M2 exon. If cleavage of the primary transcript and addition of the poly-A tail occurs at site 1, the M1 and M2 exons are lost. Excision of the introns and splicing of the remaining exons then produces mRNA encoding the secreted form of the heavy chain. If cleavage and polyadenylation of the primary transcript occurs instead at site 2, then a different pattern of splicing results. In this case, splicing removes the S sequence at the 3_ end of the C_4 exon, which encodes the hydrophilic carboxyl-terminal end of the secreted form, and joins the remainder of the C_4 exon with the M1 and M2 exons, producing mRNA for the membrane form of the heavy chain. Later DNA sequencing revealed that all the CH gene segments have S sequence and two additional downstream M1 and M2 exons that encode the transmembrane and cytoplasmic segments. Thus, differential processing of a common primary transcript determines whether the secreted or membrane form of an immunoglobulin will be produced.
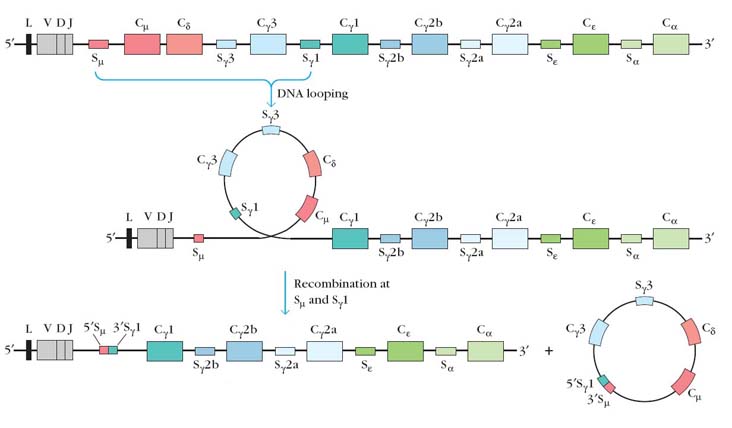
CLASS SWITCHING:
Class switching refers to the change in the constant region of heavy chain Isotype of antibody from plasma cell i.e. one Isotype is changed to another Isotype. Because of this, this process is otherwise known as Isotype switching. For example: mature B cell after encountering antigen differentiated into plasma and memory cell. Then plasma cell produce secretary antibodies of Ig M type initially. After some time, the same plasma cell starts to secrete another isotypic antibodies like Ig G or Ig A or Ig E. This is known as class switching. The mechanism for class switching is the DNA rearrangement by the process of DNA looping. Class switching depends upon the interplay of three elements: switch regions, a switch recombinase, and the cytokine signals that dictate the isotype to which the B cell switches. Switch regions located 23 kb upstream from each CH segment except for delta chain. These switch regions, though rather large (2 to 10 kb), are composed of multiple copies of short repeats (GAGCT and TGGGG). Switch recombinase recognize these regions and carry out switching. Cytokines known as switch factors and act as primary determinant of secretary isotype. For example IL-4 results in class switch form Ig M to Ig G or Ig E.
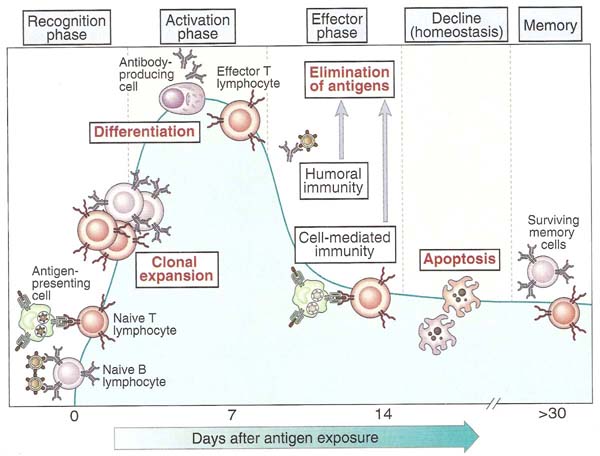
LYMPHOCYTE DIFFERENTIATION:
Lymphocyte differentiation is the process through which the functional T and B cells are produced i.e. effector and memory cells. This process occurs after lymphocytes encounter antigen. This process is preceded by antigen recognition, activation and proliferation of lymphocytes. B cell differentiation already discussed.
Maturation of T cell:
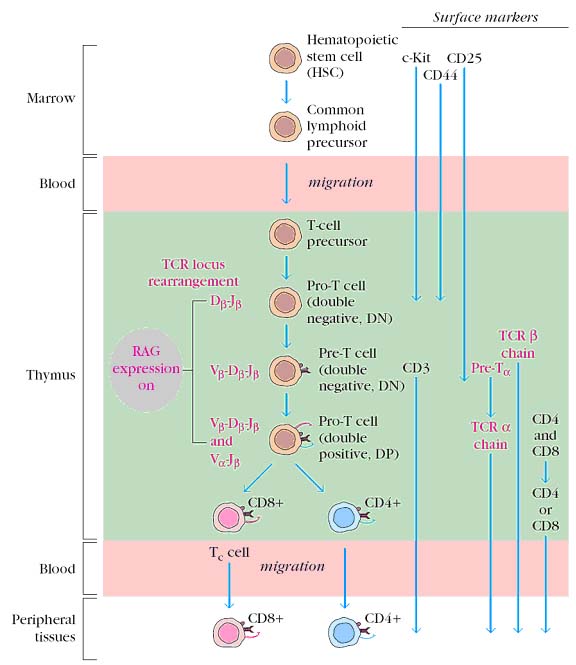
Proliferation and differentiation:
Pre-T cells were formed from pluripotent stem cells in bone marrow and they migrate to thymus. In thymus, Pre-T cells matured into mature TH and TC cells. Maturation process depends upon the expression of TCR and some other cell surface components. Different T cell populations vary in their cell surface components and functions. Matured T cell then move to peripheral lymphoid organs for antigen encounter.
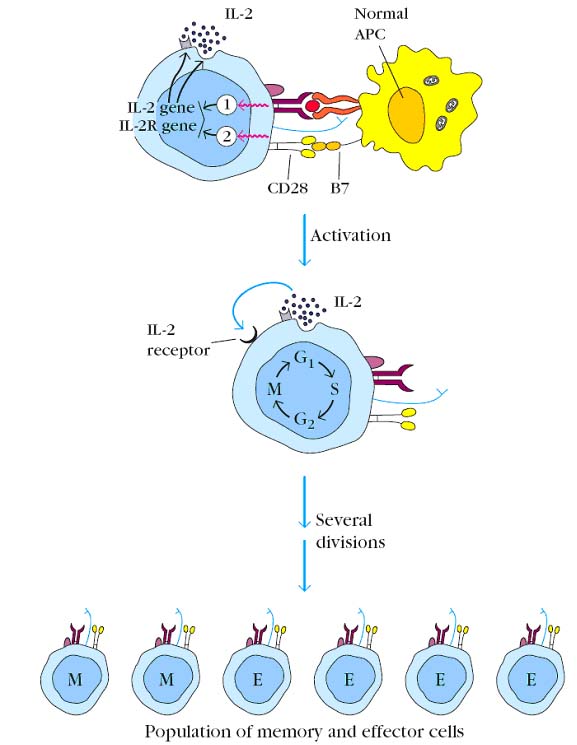
Since T cells are MHC restricted cells, they recognize antigen only through MHC Antigen complex i.e. they recognize only processed and presented antigen through APC cells. If a naive T cell recognizes an antigen-MHC complex on an appropriate antigen-presenting cell or target cell, it will be activated, initiating a primary response. About 48 hours after activation, the naive T cell enlarges into a blast cell and begins undergoing repeated rounds of cell division. Activation depends on a signal induced by engagement of the TCR complex and a co-stimulatory signal induced by the CD28-B7 interaction. These signals trigger entry of the T cell into the G1 phase of the cell cycle and, at the same time, induce transcription of the gene for IL-2 and the alpha chain of the high-affinity IL-2 receptor. In addition, the co-stimulatory signal increases the half-life of the IL-2 mRNA. The increase in IL-2 transcription, together with stabilization of the IL-2 mRNA, increases IL-2 production by 100-fold in the activated T cell. Secretion of IL-2 and its subsequent binding to the high-affinity IL-2 receptor induces the activated naive T cell to proliferate and differentiate. T cells activated in this way divide 23 times per day for 45 days, generating a large clone of progeny cells, which differentiate into memory or effector T-cell populations.
The various effector T cells carry out specialized functions such as cytokine secretion and B-cell help (activated CD4_ TH cells) and cytotoxic killing activity (CD8_ CTLs). Effector cells are derived from both naive and memory cells after antigen activation. Effector cells are short-lived cells, whose life spans range from a few days to a few weeks. The effector and naive populations express different cell-membrane molecules, which contribute to different recirculation patterns. Effector TH cells consists of subsets that produce distinct sets of cytokines and perform different functions such as TH1 and TH2 subsets. TC cells differentiate into functional CTLs, with the ability to kill target cells that express the peptide-MHC complexes these T cells recognize. The differentiation of T cell is associated with the transcriptional activation of genes encoding effector molecules. Some of these effector molecules, such as cytokines and CTL granule proteins, are released from the T cell, and others such as CD40 ligand (CD40L) and Fas ligand are cell surface molecules that are involved in effector functions.
The memory T-cell population is derived from both naοve T cells and from effector cells after they have encountered antigen. Memory T cells are antigen-generated, generally long-lived, quiescent cells that respond with heightened reactivity to a subsequent challenge with the same antigen, generating a secondary response. Like naive T cells, most memory T cells are resting cells in the G0 stage of the cell cycle, but they appear to have less stringent requirements for activation than naive T cells do. For example, naive TH cells are activated only by dendritic cells, whereas memory TH cells can be activated by macrophages, dendritic cells, and B cells. It is thought that the expression of high levels of numerous adhesion molecules by memory TH cells enables these cells to adhere to a broad spectrum of antigen-presenting cells. Memory cells also display recirculation patterns that differ from those of naive or effector T cells. Memory T cells appear to be heterogeneous in their migration and functional capabilities. In humans, memory T cells consist of subsets that differ in the expression of the chemokine receptor CCR7. It is believed that CCR7-high memory cells preferentially migrate to lymph nodes and proliferate rapidly on repeated encounter with antigen, providing a large pool of T cells in secondary immune response. CCR7-low memory cells produce effector cytokines and they may migrate preferentially to peripheral sites of infection and inflammation, where they function to eliminate antigens.
Clonal anergy: It is a proposed mechanism for rendering peripheral antigen-reactive lymphocytes functionally inactive. It results in failure in protection against specific antigen.
Clonal deletion: It is a proposed mechanism for eliminating, by apoptosis, self-reactive lymphocytes induced by their contact with self-antigens during selection process of maturation. It results in the prevention of autoimmune disorders. It also responsible for returning the immune system to a state of rest or homeostasis after the antigen is eliminated.
Clonal expansion: It is a proposed mechanism for lymphocyte proliferation after lymphocytes activated by APC or target cell. It results in rise of specific lymphocyte population.
T CELL AND B CELL INTERACTION:
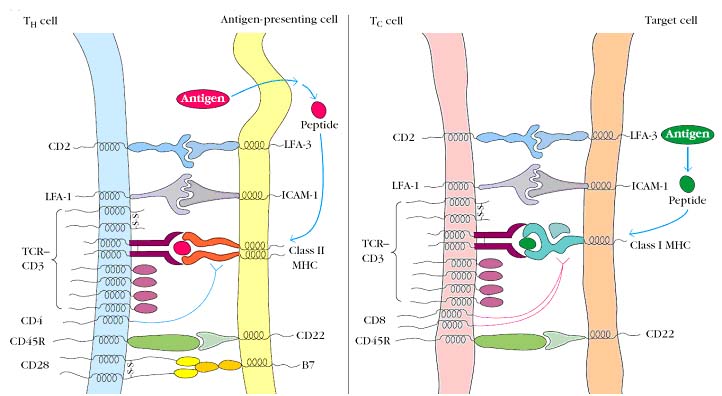
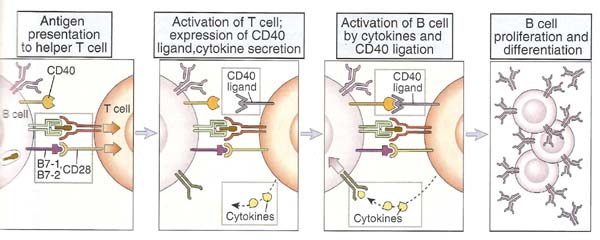
T - cell ad B - cell interaction found to be mutually supportive i.e. binding of B - cell to TH - cell activates B - cell in the case of activation of T cell dependent antigen recognition whereas TH cell is activated when B cell act as APC. The interaction between helper T cells and B lymphocytes sequentially involves antigen induced activation of the two cell types. First physical contact between the cells occurs, and then antigen presented by B cell to differentiated helper T cells. This activates T cell which intern induces expression of membrane and secreted molecules. These molecules bind to B cell and activate B cells.
Generally helper T cell and B cell interaction occurs in peripheral lymphoid organs. It is well studied in lymph node where interaction proved through immunohistochemical analysis. It usually occurs in T cell zone near to B cell zone.
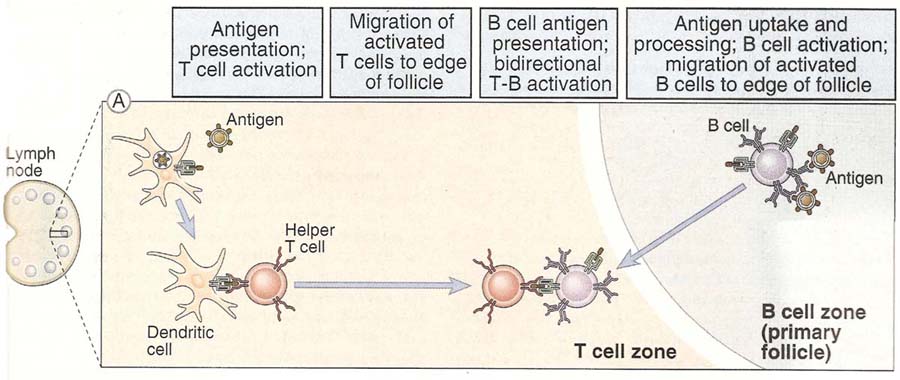
T cell and B cell interaction can be studied in two different headings first antigen presentation by B cell to T cell and second B cell activation by T cell.
1. Antigen presentation by B cell:
Antigen specific B lymphocytes bind the native antigen to membrane Ig molecules, internalize and process it in endosomal vesicles and present on their surfaces peptide fragments of the antigen complexed with class II MHC molecules. Antigen binding to membrane Ig enhances the expression of costimulators that increase the ability of the B lymphocytes to activate helper T cells. This occurs at the same time that antigen is being processed and presented by the B cell. The principal costimulators that are expressed on activated B cells are B7-2 and B7-1, both of which bind to CD28 on the T cell. Helper T cells can then recognize peptide-MHC complexes (signal 1) and costimulators (signal 2) and are stimulated to perform their effector function which is to promote B cell growth and differentiation. Thus the B cells themselves function as APCs in humoral immune responses to protein antigens. The peptide-MHC complexes can then be recognized by specific CD4+ helper T lymphocytes.
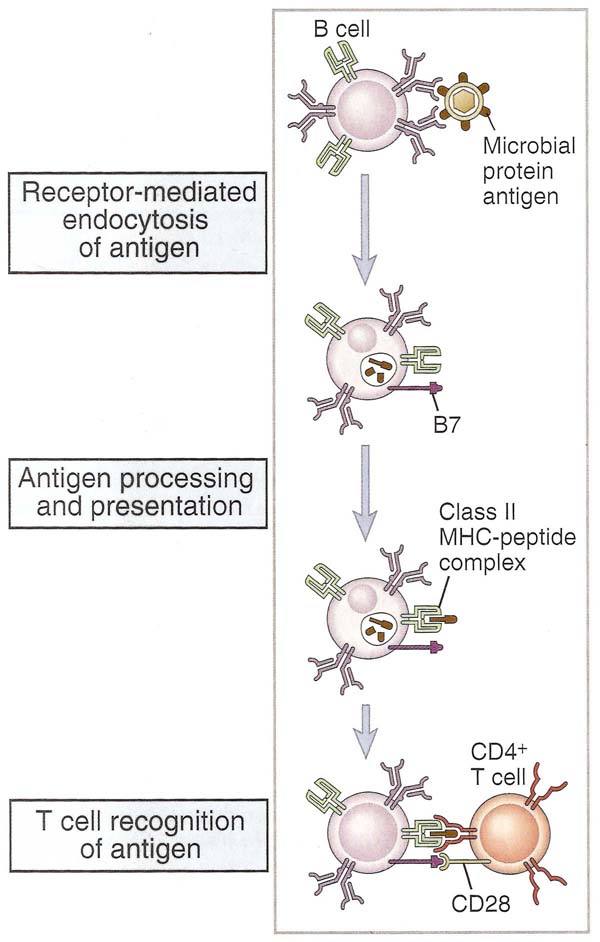
2. B cell activation by T cell:
After antigen presented by B cell, T cells express CD40 ligand (CD40L) on its surface and release cytokines. CD40L is a T cell membrane protein that is structurally homologous to TNF and Fas ligand. CD40L interacts with CD40 on B cell when T cell and B cell binds. CD40L binding to CD40 results in oligomerization of CD40 molecules and this induces the association of cytoplasmic proteins called TNF receptor associated factors (TRAFs) to the cytoplasmic domain of CD40. The TRAFs recruited to CD40 initiate enzyme cascades that lead to the activation and nuclear translocation of transcription factors, including NF-kb and AP-1. Activated helper T cells were secret cytokines that act in concert with CD40L to stimulate B cell proliferation and production of antibodies of different isotypes.