ORGANS OF IMMUNE SYSTEM
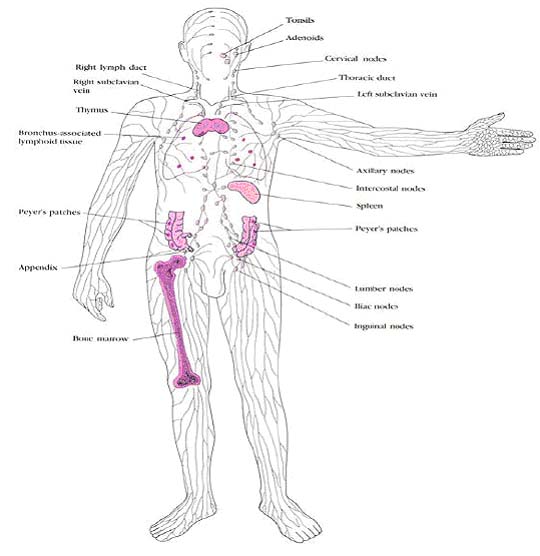
The lymphoid organs are organized tissues containing large numbers of lymphocytes in a framework of nonlymphoid cells. In these organs, the interactions lymphocytes make with nonlymphoid cells are important either to lymphocyte development, to the initiation of adaptive immune responses, or to the sustenance of lymphocytes. Lymphoid organs can be divided broadly into central or primary lymphoid organs, where lymphocytes are generated, and peripheral or secondary lymphoid organs, where adaptive immune responses are initiated and where lymphocytes are maintained. The central lymphoid organs are the bone marrow and the thymus, a large organ in the upper chest; the location of the thymus, and of the other lymphoid organs, is shown schematically in the following figure:

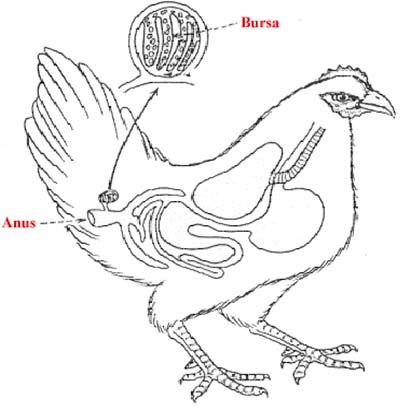
Lymphocytes (T and B cells) are found in the circulation and in lymphoid organs and tissues. Primary lymphoid organs provide a site for lymphocytes to develop from a lymphoid stem cell. This cell arises like other hematopoietic cells from stem cells in the bone marrow and then enters the circulation. In birds, B cells mature in the Bursa of Fabricius which is embryologically and morphologically similar to the thymus. T lymphocytes develop in the thymus. The thymus is found in the anterior mediastinum and becomes smaller with age. The thymus is where T cells are "educated" to distinguish self from nonself.
B cells develop in the bone marrow which is considered a primary lymphoid organ. The bone marrow also acts as a secondary organ because it contains some antibody-secreting plasma cells as well. Secondary lymphoid organs and tissues are the sites where lymphocytes are most active and it is the site were antigen encounters immune cells. The white pulp of the spleen is the common site for plasma cells to develop. Macrophages are also active in the spleen red pulp; here they engulf blood-borne antigens to be presented to T lymphocytes in the circulation. Lymph nodes, also secondary tissue, are found throughout the body. These nodes contain many T and B lymphocytes arranged so that as lymph fluid enters the nodes, it is filtered through the lymphocytes. This allows antigens and microorganisms to be recognized by antigen recognition molecules on lymphocytes resulting in an immune response. The sub mucosa of the lung and gut are other important secondary lymphoid tissues. The lung bronchiole associated lymphoid tissue (BALT) is much like the gut associated lymphoid tissue (GALT). These are both mucosal sites and such tissue is often referred to as "mucosal associated lymphoid tissue" (MALT). It is in these sites that antigen is taken from the lungs or intestine. Plasma cells secreting IgA reside in these tissues. Peyer's patches are large specialized aggregations of lymphoid tissue in the gut which play a major role in the production of IgA.
CENTRAL LYMPHOID ORGANS:
T and B lymphocytes develop from the same stem cell in the bone marrow. This lymphocyte stem itself came from a leukocyte stem from which all white blood cells develop. While in the bone marrow, pre-T cells arise which reach final maturity in the thymus under the influence of thymic hormones like thymosin and thymulin etc. B cells, in mammals, come from the bone marrow reasonably mature, although they may undergo further maturation in the mucosal associated lymphoid tissue (MALT). In birds, pre-B cells leave the bone marrow and mature in a unique organ called the Bursa of Fabricius. It has a structure similar to the thymus and also secretes hormones which control the maturation process. Central lymphoid organs are otherwise known as generative lymphoid organs because of its nature to produce immune cells.
BURSA:
This is a sac like lymphoepithelial structure arising as a dorsal diverticulum from the cloaca in birds. This organ was first described by Fabricius and hence the name Bursa of Fabricius. This organ originates from the hindgut epithelium of the chick embryo at about the 15th day of development. By the time of hatching, it develops into a fully functional organ. By about 4 months of age, it reaches the maximum size of about 3 cm in diameter after which it starts involuting. The most prominent cells in bursa include lymphocytes, macrophages and plasma cells. The lymphocytes of bursa are similar to the lymphocytes of thymus in morphology but differ in function. The function of Bursa is identified through the bursectomy studies. In birds, B cells mature in the Bursa of Fabricius which is embryologically and morphologically similar to the thymus. PreB cells enter and undergo rapid proliferation during which they mature. The mature B lymphocytes later migrate to the bursa dependent regions of the secondary lymphoid organs such as spleen and lymph node. There, the lymphocytes produce plasma cells which later secrete antibodies in response to antigenic stimulus thus having major role in humoral or antibody mediated immunity.
BONE MARROW:
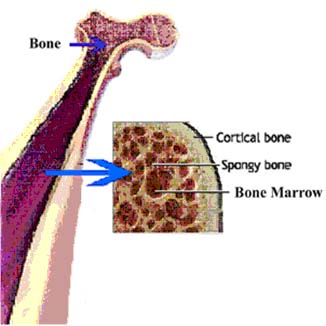
The mammalian functional equivalent of the Bursa is the bone marrow. In humans and mice, bone marrow is the site of B-cell origin and development. The soft tissue present in the cavities of bones referred as bone marrow. Bone marrow which actively involved in blood cell production appears in red. So it is called as red marrow. It usually present in young individuals. The red marrow that is found in these bones consists of a sponge like reticular framework located between long trabeculae. The spaces in this framework are filled with fat cells, stromal fibroblasts and precursors of blood cells. These precursors mature and exit through the dense network of vascular sinuses to enter the vascular circulation. In adult, much of the red marrow is replaced by fatty tissue and becomes yellow marrow.
B-cell Maturation:
Arising from lymphoid progenitors, immature B cells proliferate and differentiate within the bone marrow, and stromal cells within the bone marrow interact directly with the B cells and secrete various cytokines that are required for development. Like thymic selection during T cell maturation, a selection process within the bone marrow eliminates B cells with self-reactive antibody receptors. In humans, B cells are functionally mature when they exit the bone marrow and enter circulation. Maturation of the B cells involves a genetic rearrangement to bring the separate genes that make up the immunoglobulin heavy and light chains together. The gene rearrangements for the T cell antigen receptor and for immunoglobulin are so similar that they use the same enzymes (the products of recombination activating genes-1 & -2, RAG-1 and RAG-2). The first event of B cell maturation that signals subsequent ones is the genetic rearrangement that permits transcription of mRNA for m and d chains. The splicing is not always exact; i.e., a functional m chain is not produced. When this happens, the cell tries to rearrange the genes on the other chromosome of the pair. If it is successful, the complete m chain serves to prevent rearrangement of the paired chromosome, known as allelic exclusion. For reasons that are not totally clear, the m chain is translated first. This signals the rearrangement of the genes to make the k light chains. Again, if the first rearrangement is not successful, the cell tries again with the other chromosome of the pair. If neither of these is successful, rearrangement of the genes for the l chain is tried. If a functional immunoglobulin (heavy and light chain) is not made, the cell undergoes apoptosis since its purpose is to make antibody. IgM is expressed on the surface first, followed by IgD. If the immature B cell is exposed to an antigen to which it can bind before the IgD is expressed, the B cell dies (apoptosis is initiated). Since the antigens the immature B cell would be likely to see are those that are always in circulation; i.e., normal self antigens, this is a way to prevent development of B cells which can attack self tissues.
THYMUS:
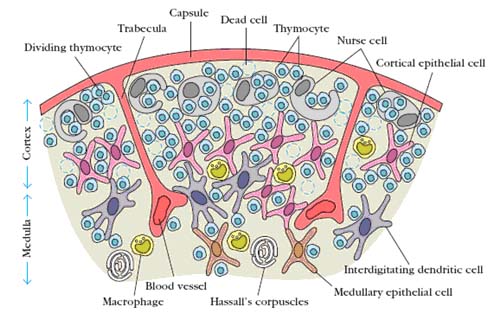
The thymus is the site of maturation of T cells. The thymus is a bilobed organ situated in the anterior mediastinum. The thymus develops from the third pharyngeal pouch. The parathyroid and major vessels in the upper thorax also develop from these pouches, so a defect in embryonic development can result is greater or lesser involvement of the parathyroid and major vessels. The thymus has two distinct lobes each of which consists of multiple lobules. The lobules have a cortex that is densely packed with rapidly dividing lymphocytes and a less dense medulla in which most of the cells are resting, mature T cells. The thymus is at its largest relative to body weight around the time of birth, but continues to grow in size until puberty. Thereafter it involutes, and the lymphoid elements are progressively replaced with fatty tissue, a process which continues throughout adulthood. PreT cells enter the thymus through the post-capillary venules located at the juncture of the cortex and medulla. The lymphocytes in the thymus called as thymocytes. Over the next several days, they migrate out into the cortex under the influence of some unknown chemotactic factor(s) then back through the cortex and into the medulla. During this time, they divide approximately every 12 hours. Under the influence of several thymic hormones, the best known of which are thymopoeitin and thymosin, they gradually acquire the membrane and functional characteristics of mature T cells. The mature T cells then leave the thymus through the postcapillary venules and enter circulation. Hassall’s corpuscles in medullary region composed of tightly packed whorls of epithelial cells that may be remnants of degenerating cell. Mouse without thymus usually referred as nude mouse because it suffers from severe infection. DiGeorge syndrome also suffers from T cell deficiency because of mutations in genes required for thymus development.
SECONDARY LYMPHOID OR PERIPHERAL ORGANS:
An immune response is initiated only when an antigen comes into contact with lymphocytes that can recognize and respond to it. To maximize contact between antigens and lymphocytes, lymphoid tissue is organized such that tissue fluids (lymph) and blood are constantly filtered through beds of lymphocytes. These beds are concentrated in areas that drain the most likely areas of access by pathogens; i.e., the extremities and the mucosal surfaces. The spleen is the lymphoid tissue which screens blood for pathogens that have escaped from the tissues whereas lymph node is the lymphoid tissue which screens lymph for pathogens.
LYMPH NODE:

Lymph nodes are small nodular aggregates of lymphocyte rich tissue situated along lymphatic channels throughout the body. Lymph node consists of an outermost cortex and an inner medulla. The cortex is composed of an outer cortex of B cells organized into lymphoid follicles, and deep, or paracortical, areas made up mainly of T cells and dendritic cells. Beneath the subcapsular sinus, the outer cortex contains aggregates of cells called follicles. Some follicles contain central areas called germinal centers, which stain lightly with commonly used histologic stains. Follicles without germinal centers are called primary follicles and those with germinal centers are secondary follicles. When an immune response is underway, some of the follicles contain central areas of intense B-cell proliferation called germinal centers and are known as secondary lymphoid follicles. These reactions are very dramatic, but eventually die out as senescent germinal centers. Lymph draining from the extracellular spaces of the body carries antigens in phagocytic dendritic cells and macrophages from the tissues to the lymph node via the afferent lymphatics. Lymph leaves by the efferent lymphatic in the medulla. The medulla consists of strings of macro-phages and antibody-secreting plasma cells known as the medullary cords. Naive lymphocytes enter the node from the bloodstream through specialized postcapillary venules and leave with the lymph through the efferent lymphatic. Afferent lymphatic vessels drain fluid from the tissues and also carry antigen-bearing cells and antigens from infected tissues to the lymph nodes, where they are trapped. In the lymph nodes, B lymphocytes are localized in follicles, with T cells more diffusely distributed in surrounding paracortical areas, also referred to as T-cell zones. Some of the B-cell follicles include germinal centers, where B cells are undergoing intense proliferation after encountering their specific antigen and their cooperating T cells.
SPLEEN:
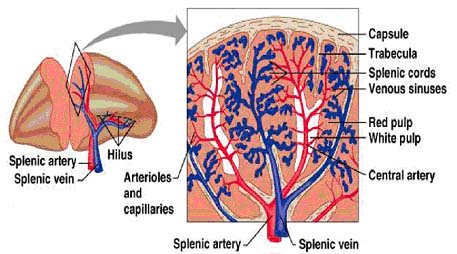
The spleen consists of red pulp, which is a site of red blood cell destruction, interspersed with lymphoid white pulp. An enlargement of a small section of the spleen shows the arrangement of discrete areas of white pulp around central arterioles. Lymphocytes and antigen- loaded dendritic cells come together in the periarteriolar lymphoid sheath (PALS). In each area of white pulp, blood carrying lymphocytes and antigen flows from a trabacular artery into a central arteriole. Cells and antigen then pass into a marginal sinus and drain into a trabecular vein. The marginal sinus is surrounded by a marginal zone of lymphocytes. Within the marginal sinus and surrounding the central arteriole is the periarteriolar lymphoid sheath, made up of T cells. The follicles consist mainly of B cells; in secondary follicles a germinal center is surrounded by a B-cell corona. Although the organization of the spleen is similar to that of a lymph node, antigen enters the spleen from the blood rather than from the lymph.
MUCOSAL ASSOCIATED LYMPHOID TISSUE (MALT):
The mucous membranes lining the digestive, respiratory, and urogenital systems have a combined surface area of about 400 m2 and are the major sites of entry for most pathogens. These vulnerable membrane surfaces are defended by a group of organized lymphoid tissues mentioned earlier and known collectively as mucosal-associated lymphoid tissue (MALT). Structurally, these tissues range from loose, barely organized clusters of lymphoid cells in the lamina propria of intestinal villi to well-organized structures such as the familiar tonsils and appendix, as well as Peyer’s patches, which are found within the submucosal layer of the intestinal lining. The functional importance of MALT in the body’s defense is attested to by its large population of antibody-producing plasma cells, whose number far exceeds that of plasma cells in the spleen, lymph nodes, and bone marrow combined. It is classified under both secondary and tertiary lymphoid organs because it act as both point of antigen encounter and entry respectively
Tonsils:
The tonsils are found in three locations: lingual at the base of the tongue; palatine at the sides of the back of the mouth; and pharyngeal (adenoids) in the roof of the nasopharynx. All three tonsil groups are nodular structures consisting of a meshwork of reticular cells and fibers interspersed with lymphocytes, macrophages, granulocytes, and mast cells. The B cells are organized into follicles and germinal centers; the latter are surrounded by regions showing T-cell activity. The tonsils defend against antigens entering through the nasal and oral epithelial routes.
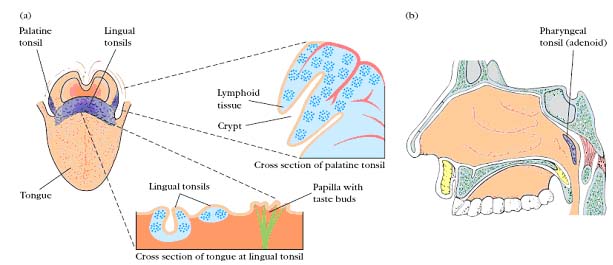
Peyer’s Patches:

The lamina propria, which lies under the epithelial layer, contains large numbers of B cells, plasma cells, activated TH cells, and macrophages in loose clusters. Histologic sections have revealed more than 15,000 lymphoid follicles within the intestinal lamina propria of a healthy child. The submucosal layer beneath the lamina propria contains Peyer’s patches, nodules of 30–40 lymphoid follicles. Like lymphoid follicles in other sites, those that compose Peyer’s patches can develop into secondary follicles with germinal centers. The epithelial cells of mucous membranes play an important role in promoting the immune response by delivering small samples of foreign antigen from the lumina of the respiratory, digestive, and urogenital tracts to the underlying mucosal-associated lymphoid tissue.
GALT (Gut Associated Lymphoid Tissue):
The gut-associated lymphoid tissues (GALT), which include the tonsils, adenoids, and appendix, and specialized structures called Peyer's patches in the small intestine, collect antigen from the epithelial surfaces of the gastrointestinal tract. In Peyer's patches, which are the most important and highly organized of these tissues, the antigen is collected by specialized epithelial cells called multi-fenestrated or M cells. The lymphocytes form a follicle consisting of a large central dome of B lymphocytes surrounded by smaller numbers of T lymphocytes. Similar but more diffuse aggregates of lymphocytes protect the respiratory epithelium, where they are known as bronchialassociated lymphoid tissue (BALT), and other mucosa, where they are known simply as Mucosal-Associated Lymphoid Tissue (MALT).
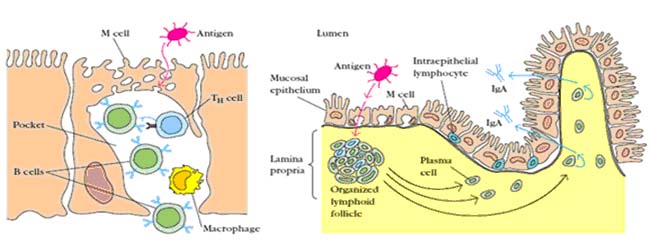
Collectively, the mucosal immune system is estimated to contain as many lymphocytes as all the rest of the body, and they form a specialized set of cells obeying somewhat different rules. The bulk of the GALT tissue is B cells, organized in a large and highly active domed follicle. T cells occupy the areas between follicles. The antigen enters across a specialized epithelium made up of so-called M cells. Although this tissue looks very different from other lymphoid organs, the basic divisions are maintained.
CUTANEOUS ASSOCIATED LYMPHOID TISSUE (CALT)[Tertiary Lymphoid Organ]:
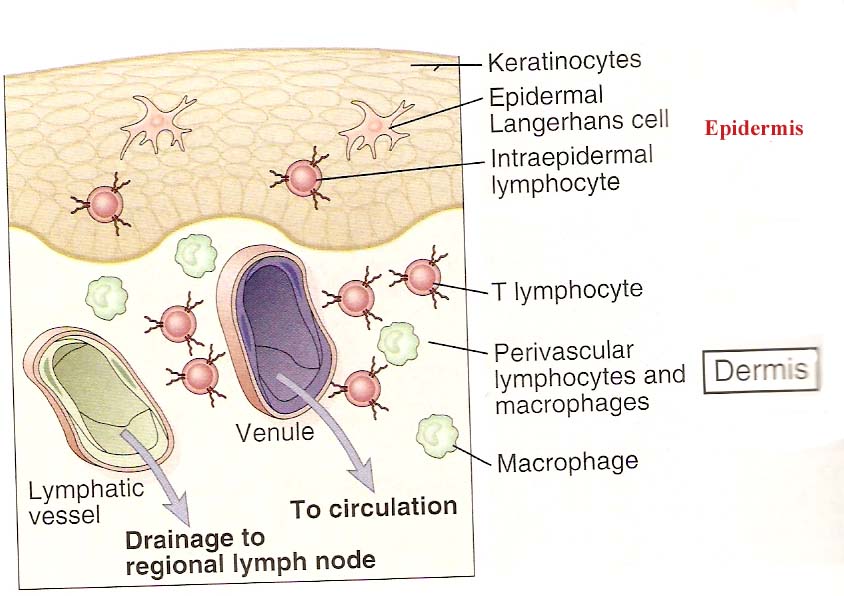
The skin is an important anatomic barrier to the external environment, and its large surface area makes this tissue important in nonspecific (innate) defenses. Many foreign antigens gain entry into the body through the skin, so that it is called as tertiary lymphoid organ. The epidermal (outer) layer of the skin is composed largely of specialized epithelial cells called keratinocytes. These cells secrete a number of cytokines that may function to induce a local inflammatory reaction. In addition, keratinocytes can be induced to express class II MHC molecules and may function as antigen-presenting cells. Scattered among the epithelial-cell matrix of the epidermis are Langerhans cells, a type of dendritic cell, which internalize antigen by phagocytosis or endocytosis. The Langerhans cells then migrate from the epidermis to regional lymph nodes, where they differentiate into interdigitating dendritic cells. These cells express high levels of class II MHC molecules and function as potent activators of naive TH cells. The epidermis also contains so-called intraepidermal lymphocytes. These are similar to the intraepithelial lymphocytes of MALT in that most of them are CD8_ T cells, many of which express _ T-cell receptors, which have limited diversity for antigen. These intraepidermal T cells are well situated to encounter antigens that enter through the skin and some immunologists believe that they may play a role in combating antigens that enter through the skin. The underlying dermal layer of the skin contains scattered CD4_ and CD8_ T cells and macrophages. Most of these dermal T cells were either previously activated cells or are memory cells.
LYMPHOCYTES CIRCULATION BETWEEN LYMPH AND BLOOD:
Small B and T lymphocytes that have matured in the bone marrow and thymus but have not yet encountered antigen are referred to as naive lymphocytes. These cells circulate continually from the blood into the peripheral lymphoid tissues, which they enter by squeezing between the cells of capillary walls. They are then returned to the blood via the lymphatic vessels or, in the case of the spleen, return directly to the blood. In the event of an infection, lymphocytes that recognize the infectious agent are arrested in the lymphoid tissue, where they proliferate and differentiate into effector cells capable of combating the infection.
When an infection occurs in the periphery, for example, large amounts of antigen are taken up by dendritic cells which then travel from the site of infection through the afferent lymphatic vessels into the draining lymph nodes. In the lymph nodes, these cells display the antigen to recirculating T lymphocytes, which they also help to activate. B cells that encounter antigen as they migrate through the lymph node are also arrested and activated, with the help of some of the activated T cells. Once the antigen-specific lymphocytes have undergone a period of proliferation and differentiation, they leave the lymph nodes as effector cells through the efferent lymphatic vessel.
Because they are involved in initiating adaptive immune responses, the peripheral
lymphoid tissues are not static structures but vary quite dramatically depending upon whether or not infection is present. The diffuse mucosal lymphoid tissues may appear in response to infection and then disappear, whereas the architecture of the organized tissues changes in a more defined way during an infection. For example, the B-cell follicles of the lymph nodes expand as B lymphocytes proliferate to form germinal centers, and the entire lymph node enlarges, a phenomenon familiarly known as swollen glands.
CELLS OF THE IMMUNE SYSTEM
HEMATOPOEISIS:
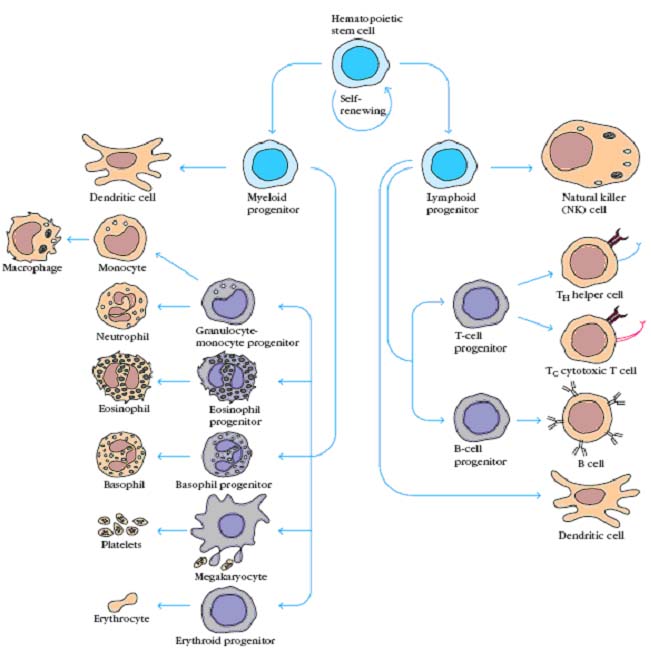
All the cells of the immune system are derived from pluripotent stem cells in the bone marrow by a process called hematopoeisis.
These pluripotent cells divide to produce two more specialized types of stem cells, a common lymphoid progenitor that gives rise to the T and B lymphocytes responsible for adaptive immunity, and a common myeloid progenitor that gives rise to different types of leukocytes (white blood cells), erythrocytes (red blood cells that carry oxygen), and the megakaryocytes that produce platelets that are important in blood clotting. T and B lymphocytes are distinguished by their sites of differentiation T cells in the thymus and B cells in the bone marrow and by their antigen receptors. Mature T and B lymphocytes circulate between the blood and peripheral lymphoid tissues. After encounter with antigen, B cells differentiate into antibody-secreting plasma cells, whereas T cells differentiate into effector T cells with a variety of functions. Third lineages of lymphoid-like cells, the natural killer cells, derive from the same progenitor cell but lack the antigen-specificity that is the hallmark of the adaptive immune response. The leukocytes that derive from the myeloid stem cell are the monocytes, the dendritic cells, and the basophils, eosinophils, and neutrophils. The latter three are collectively termed either granulocytes, because of the cytoplasmic granules whose characteristic staining gives them a distinctive appearance in blood smears, or polymorphonuclear leukocytes, because of their irregularly shaped nuclei. They circulate in the blood and enter the tissues only when recruited to sites of infection or inflammation where neutrophils are recruited to phagocytose bacteria. Eosinophils and basophils are recruited to sites of allergic inflammation, and appear to be involved in defending against parasites. Immature dendritic cells travel via the blood to enter peripheral tissues, where they ingest antigens. When they encounter a pathogen, they mature and migrate to lymphoid tissues, where they activate antigen-specific T lymphocytes. Monocytes enter tissues, where they differentiate into macrophages; these are the main tissue-resident phagocytic cells of the innate immune system. Mast cells arise from precursors in bone marrow but complete their maturation in tissues; they are important in allergic responses.
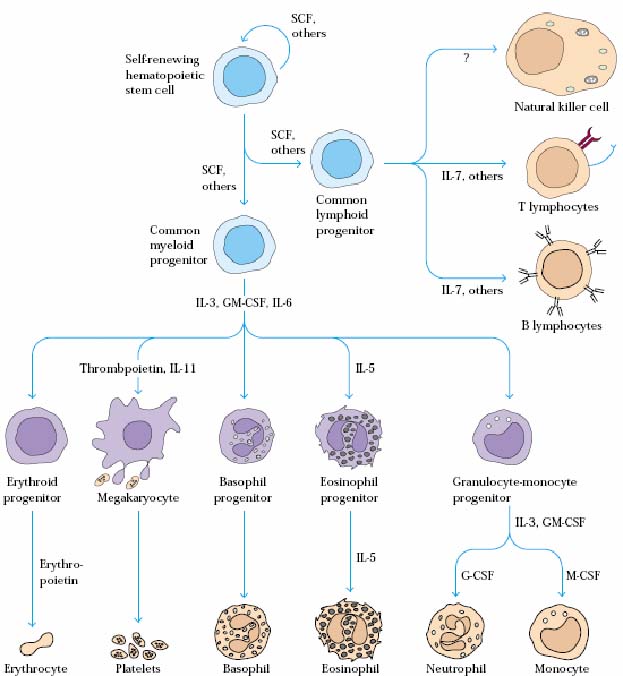
In bone marrow, hematopoietic cells grow and mature on a meshwork of stromal cells, which are nonhematopoietic cells that support the growth and differentiation of hematopoietic cells. Stromal cells include fat cells, endothelial cells, fibroblasts, and macrophages. Stromal cells influence the differentiation of hematopoietic stem cells by providing a hematopoietic-inducing microenvironment (HIM) consisting of a cellular matrix and factors that promote growth and differentiation. Many of these hematopoietic growth factors are soluble agents that arrive at their target cells by diffusion, others are membrane-bound molecules on the surface of stromal cells that require cell-to-cell contact between the responding cells and the stromal cells. During infection, hematopoiesis is stimulated by the production of hematopoietic growth factors by activated macrophages and T cells. Various growth factors are required for the survival, proliferation, differentiation, and maturation of hematopoietic cells in culture. These growth factors, the hematopoietic cytokines, are identified by their ability to stimulate the formation of hematopoietic cell colonies in bone-marrow cultures. Among the cytokines detected in this way was a family of acidic glycoproteins, the colony-stimulating factors (CSFs), named for their ability to induce the formation of distinct hematopoietic cell lines. Another important hematopoietic cytokine detected by this method was the glycoprotein erythropoietin (EPO). Produced by the kidney, this cytokine induces the terminal development of erythrocytes and regulates the production of red blood cells.
MYELOID CELLS:
NEUTROPHILS:
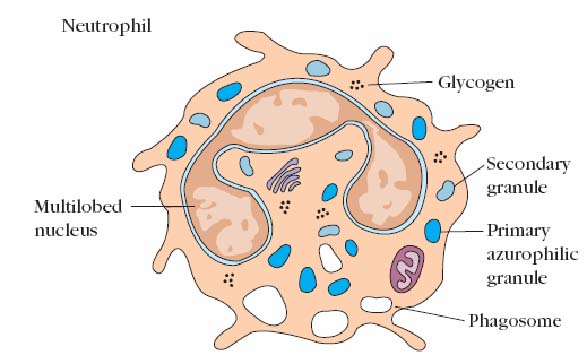
Neutrophils are produced in the bone marrow from the granulocyte-monocyte stem cell. These cells are often called polymorphonuclear cells (PMN's). This is because of the polymorphic shape of the nucleus. Sometimes the terms neutrophil and PMN are used interchangeably. The neutrophil's main role is in inflammation. They are the first cells to arrive at the site of inflammation by leaving the blood, through the endothelium into the tissue (extravasation). Neutrophils are attracted into the tissue by chemotactic factors that include complement proteins, clotting proteins (stimulated to be produced by tissue damage) and T cell derived cytokines. In the tissues, neutrophils are active phagocytic cells, like macrophages. Neutrophils, however, do not act as antigen presenting cells. Neutrophils, instead, are most effective at killing ingested microorganisms and can do this by oxygen dependent or independent pathways. Neutrophils exhibit a larger respiratory burst than macrophages and consequently are able to generate more reactive oxygen intermediates and reactive nitrogen intermediates. It is constitute about 50 – 70 % of leucocyte population in blood. It consists of two types of granules. The larger, denser primary granules are a type of lysosome containing peroxidase, lysozyme, and various hydrolytic enzymes. The smaller secondary granules contain collagenase, lactoferrin, and lysozyme. Both primary and secondary granules fuse with phagosomes, whose contents are then digested and eliminated much as they are in macrophages.
EOSINOPHILS:
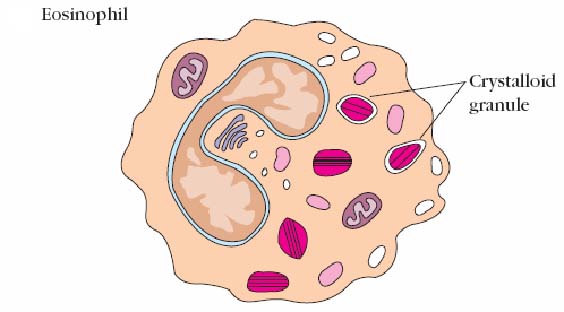
Eosinophils are granulocytes named because of their intense staining with 'eosin'. Eosinophils make up about 1-3% of leukocytes in circulation. They are similar in appearance to neutrophils except the nucleus is usually bilobed and their granules take up eosin. Under the microscope, eosinophils typically have a bilobed nucleus and contain many basic crystal granules in their cytoplasm. The granules are lysosomes in that they contain hydrolytic enzymes, peroxidase, and catalyze. Eosinophils may help limit the inflammation induced by basophils and mast cells in that they contain histaminase. About half of the material in the granules is major basic protein. They also contain aryl sulfatase. The latter two substances are toxic to parasitic worms (helminthes). Indeed, eosinophils appear to be the major host defense against these invaders. The granules are eosinophil mediators that are toxic to many organisms and also to tissues as in asthma. Eosinophils are motile and phagocytic and are particularly active in parasitic infection. In fact, blood work showing an increase in eosinophils is often caused by parastic diseases. As the parasites are very big to be phagocytosed, on appropriate stimulation, the eosinophils degranulate. Degranulation involves the fusion of the intracellular granules with the plasma membrane and the components are released to the outside of the cell, thereby killing the parasite. Since parasites aren't very common in Canada, eosinophilia may also be due to allergic disease like asthma, hay fever etc.
BASOPHILLS AND MASTCELLS:
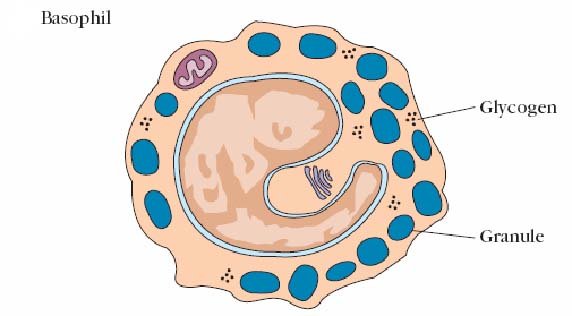
These two cells have similar functions, but must be considered distinct because of their different developmental histories. Basophils are found in circulation where they comprise about 0.2% of the leukocytes. They are rounded cells about 8-10 in diameter. They have an elongated nucleus usually with two constrictions which is sometimes folded into an S shape. Mast cells are often elongated. Those from connective tissues seem to be somewhat different from mucosal mast cells. For example, the latter require the presence of TH cells or their products for proliferation whereas connective tissue mast cells do not. Both mast cells and basophils contain large numbers of cytoplasmic granules that take up basic dyes. They are so numerous that they obscure other cell organelles. These granules contain large amounts of heparin and eosinophil chemotactic factor of anaphylaxis (ECF-A) and lesser amounts of histamine, serotonin, and precursors of prostaglandins and leukotrienes. Basophils and mast cells have receptors for both C3a and C5a and for the Fc piece of IgE. Binding of C3a and C5a or cross-linking of membrane bound IgE by allergens induces release 60% - 80% of the granules of both cell types. These factors cause contraction of endothelial cells and vasodilation of capillaries resulting in the redness, warmth and fluid accumulation in tissues characteristic of inflammation. Systemic release (basophils) can cause anaphylaxis. Release of ECF-A attracts eosinophils to the area which seems to be of particular importance in combating parasitic infestations. Mast cells are formed in the tissue from undifferentiated precursor cells released into the blood from the bone marrow. They are not the tissue counterparts of basophils but they have a similar importance in allergic reactions and are only found in tissues. There are two types of mast cells found either in the connective tissues or in mucosal sites. Both types contain numerous granules with preformed mediators which can be released from mast cells after stimulation. The preformed mediators include histamine and other pharmacologically active substances. Stimulation also results in the production of newly formed mediators by mast cells such as prostaglandins and leukotrienes. Stimulation of mast cells occurs in several ways such as by the anaphylatoxins (C3a, and C5a) of the complement system or by the crosslinking of surface IgE. Mast cells have high affinity Fc receptors for the IgE that is produced against an allergen. As a result, mast cell release is most significant in either acute inflammation or in allergic responses.
MONOCYTES AND MACROPHAGES:

Monocytes circulate in the blood after leaving the bone marrow. Monocytes usually circulate in the blood for only a day or so before they enter the tissue to mature into macrophages. Monocyte production and release from the bone marrow is increased during an immune response. In addition, monocytes enter the tissues as resident cells in various locations. These fixed, resident macrophages play an important role in keeping the tissues clear of antigen and debris. More monocytes can be recruited as needed to these and other sites.
When monocytes enter the tissues and become macrophages they undergo several changes. The cells enlarge and increase the amount of intracellular lysosome allowing greater phagocytosis. In the tissues, macrophages live for months or years and may be motile. Macrophages move with amoeboid movements using pseudopods. Macrophages are usually in the resting state unless activated during an immune response. Activation of these cells may happen in response to TH derived cytokines (especially IFN gamma). Phagocytosis of antigens may also stimulate activation. Activated macrophages have an important role in phagocytosis. In this role they recognize and remove unwanted particulate matter including products of inflammation and invading organisms, antigens and toxins. Macrophages also have an important role in the presentation of antigens (as APC's) to T cells and in activating T cells with IL-1 release. Finally, macrophages have an important secretory role. After activation, these cells secrete interferons (for anti-viral activity), lysozyme and other factors that upregulate the inflammatory response. In chronic inflammation, macrophages act as scavengers and also become giant cells in the formation of granulomas. Fixed macrophages serve different functions in different tissues and are named to reflect their tissue location like alveolar macrophage in the lung, Histiocytes in connective tissues, Kupffer cells in liver, Mesangial cells in the kidney and Microgilial cells in the brain.
LYMPHOID CELLS (LYMPHOCYTES):
Resting (non-dividing) lymphocytes are small (about 8 microns diameter), round cells with a large, centrally located nucleus and a very thin rim of cytoplasm. They normally comprise from 20 - 40% of leukocytes in circulation. They have a minimal amount of metabolic activity, barely enough to stay alive. They become active, cycling cell only after interaction with an antigen or mitogen. After several rounds of cell division, lymphocytes differentiate into effector cells which act to eliminate or inactivate the invader. In the case of B cells, the effector cells are plasma cells whose main job is to produce immunoglobulin. Other cells are important in the immune response primarily because of how they affect or are affected by lymphocytes and/or their products. Lymphocytes can be divided into two major like B – Lymphocyte and T- Lymphocyte and one minor Null cell functional subgroups. The major subgroups mature in different organs.
B-CELLS:
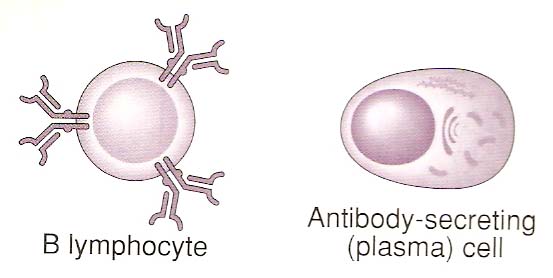
B cells develop from stem cells in the bone marrow. At the youngest stages in the bone marrow, these cells develop antigen-specific surface antibody body (IgM and IgD). The naive B cells then enter the circulation and travel around the blood and lymphatics through tissue and lymphoid organs. The B cells are called naive because they have not seen antigen yet. It is important to know that each and every B cell has a different antibody on its surface. In addition, once a B cell makes surface IgM it can then make a different class of antibody which is known as class switching but all the antibodies made by that cell recognize the same antigen. In other words, the Fab region of the antibody doesn't change, only the Fc region which defines the class. The B-cell antigen receptor (BCR) is a membrane-bound form of the antibody that the B cell will secrete after activation and differentiation to plasma cells. Antibody molecules as a class are known as immunoglobulins, usually shortened to Ig, and the antigen receptor of B lymphocytes is therefore also known as membrane immunoglobulin (mIg). In the lymph nodes, naive B cells may encounter an antigen recognized by their surface antibodies. As you might imagine, there are many (more than 90%) that circulate their entire life span without encountering antigen. These cells die within a few days. That's not nearly as exciting as what happens to B cells that do encounter antigen. The results are B cell activation, B and T cell interaction, Differentiation into plasma cells, Antibody secretion and class switching. The secondary response is dependant on a population of long-lived B memory cells. These cells are generated in lymphoid tissue after B cell activation and proliferation and reside in the bone marrow, lymph nodes and spleen. They express high affinity surface immunoglobulins which enable them to be activated by lower levels of antigen than naïve B cells. There are several diseases that involve antibodies and B cells. Selective IgA deficiency, for example, is a common asymptomatic immunodeficiency occurring in 1/600 individuals. Fewer individuals are affected more severely by malignancies of the immune system, and of the B cell line which result in Leukemias and lymphomas.
T-CELLS:
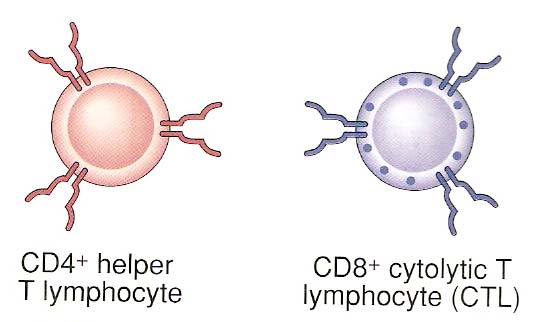
T cells are lymphocytes which develop in the thymus. There are two subpopulations of T cells (CD8+ or CD4+) that develop and their development in the thymus can be traced by surface markers. The youngest T cells have a T cell receptor associated with a protein called CD3. In the thymus, the cells then develop both a CD4 and a CD8 marker. These cells are referred to as double positive. Eventually the cells lose either CD4 or CD8 to become one of the functional subsets. The cells with a CD4 marker are called helper T cells (TH cells). The CD8 positive cells that develop are cytotoxic T cells (Tc cells). TH and Tc cells both have a TCR, but they perform very different functions in the immune system.
TH-CELL:
The generation of an immune response both humoral by B cells and cell-mediated by Tc cells (CD8+) depends on the activation of TH cells. The importance of these CD4+ cells has become obvious as these are the cells affected in AIDS (acquired immune deficiency syndrome). Mature B cells that have already seen antigen require contact with a T cell in order to become plasma cells or memory cells (T -B cell interaction). The T cells provide signals to the B cell through contact of the TCR complex and MHC-antigen, In addition, the activated T cell produces cytokines such as IL-2 and IL4, 5, 6 or IFN g which stimulate B cell proliferation and differentiation into antibody secreting B cells. The type of cytokines produced by the T cells helps the plasma cells to produce different classes of antibodies. So far it is unclear what causes this to happen, but we do know that there are two subtypes of TH cells namely TH1 and TH2 that produce different cytokines i.e. g IFN, TNF and IL 2 and IL 2,4,5,6 and 10 respectively and promote a different type of immune response. The two subsets look the same and have the same T cell markers and receptors. However, they secrete very different cytokines upon activation. In addition to other cytokines, the TH1 subset produces great amounts of IL-2 and IFN gamma; cytokines particularly important in cell mediated immunity. The TH2 subset is very good at providing B cell help by secreting IL-4,-5 and 6. Because the cytokines have different effects in an immune response, sometimes activation of one subset may be preferable to the other. For example, virus infection is best combated with interferon's anti-viral activity and cytotoxic T cells. This response is more likely to be stimulated by TH1 cytokines. The cytokines produced by the two subsets also have a cross-regulatory role. In other words, an activated TH2 cell secreting IL-4,-5, and 6 will down regulate the TH1 cells in the neighborhood. Likewise, TH1 released cytokines down regulate the TH2 responses.
T-cell activation:
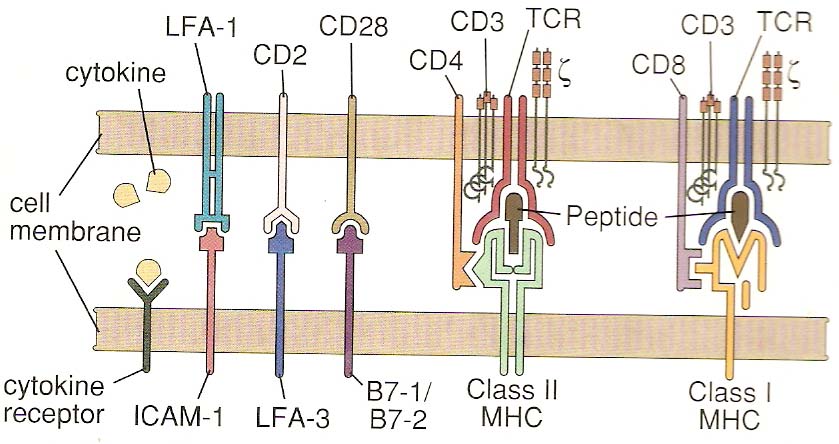
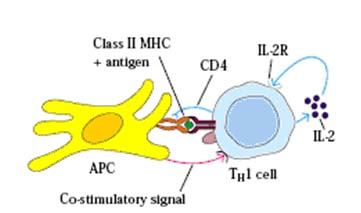
T cell activation is, in general, similar for both TH and TC cells but each are considered separately for clarity. TH cell activation is initiated by the interaction of TCR-CD3 complex with antigen-MHC class II molecules on the surface of an antigen presenting cell. This interaction initiates a cascade of biochemical events in the T cell that eventually results in growth and proliferation of the T cell. This occurs primarily through an increase in IL-2 secretion by the T cell and an increase in IL-2 receptors on the T cell surface. IL-2 is a potent T cell growth cytokine which, in T cell activation, acts in an autocrine fashion to promote the growth, proliferation and differentiation of the T cell recently stimulated by antigen. The T cell receptor is an antigen recognition molecule and therefore the T cell that best responds to the antigen presented is the one that gets turned on. Naive, bystander T cells have no IL-2 receptors and thus cannot be involved. The activation takes place through the T cell receptor complex and is aided by the CD4 molecule on TH cells and possibly other accessory molecules, such as CD45, CD28 and CD2. These molecules are accessory, but the APC also provides a co-stimulatory signal. The primary signal is the antigen-MHC II and TCR-CD3 interaction (and accessory molecules) while the co-stimulatory signal occurs with cytokines (IL-1 or IL-12). Activated TH cells then continue to become effector cells whose role includes B cell help and cytokine production.
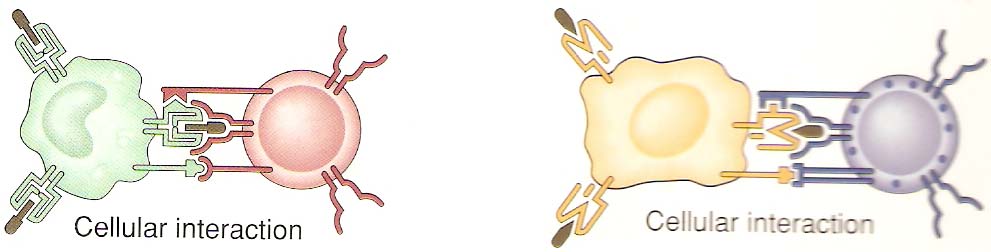
TC CELLS:
Cytotoxic T cells (Tc) are derived from a lymphocyte stem cell matured in the thymus. These cells are characterized by the presence of the CD8 marker on their surface, and an antigen-specific T cell receptor which recognizes antigens in the context of MHC class I. The main role of the cytotoxic T cell, as the name suggests, is to kill other cells. The requirement for MHC class I on the target cell means that the Tc cell is very important in recognizing and destroying self-cells that have been altered or infected. The Tc cell must first become activated and mature into a cytotoxic T lymphocyte (CTL).
Activation of the resting Tc cell is a two step process. First, the TCR on the CD8+ cell must interact with an antigen-MHC (class I) on the surface of a target cell. Secondly, the CD8+ Tc cell must be stimulated by cytokines IL-2 especially. The IL-2 is probably supplied by activated TH cells. Resting Tc does not express IL-2 receptors, but antigen stimulation increases the expression of IL-2 receptors on the surface on the Tc cell. This ensures that only the cells recognizing the antigen will become activated.
Cytotoxic T lymphocyte Activity:
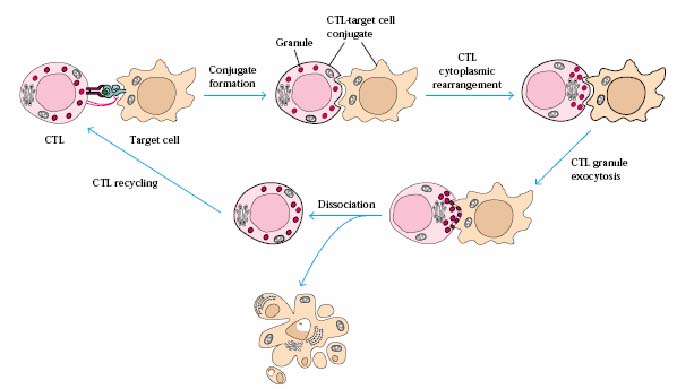
Activated CD8+ T cells (CTL's) are very effective at destroying target cells, especially virus-infected cells and tumor cells. The killing happens in three steps:
· conjugate formation between the CTL and the target cell
· Membrane attack on the target cell
· Dissociation of the two cells and target cell death
Conjugate formation:
Through the interaction of the TCR-CD3 complex and the MHC I -antigen, the target cell and the CTL form a conjugate. After antigen stimulation, the CTL receives other signals from the target cell through binding of accessory molecules and receptors.
Membrane attack:
Immediately following the formation of the conjugate, granules in the CTL start to move through the cytoplasm towards the end of nearest the target cell. The CTL changes shape and becomes flattened towards the target allowing an area of contact between the two cells. Contents of the granules are released from the CTL by exocytosis and are responsible for initiated membrane damage to the target cell. The granules release cytokines, enzymes like granzymes and a molecule called perforin that is able to poke holes in the target cell membrane.
Target Cell Death:
In addition to the perforin-mediated damage to the target cells, the CTL's have other mechanisms for attacking the cells. Soon after the CTL contact, the target cells are found to undergo apoptosis (programmed cell death). Exactly how the CTL's induce apoptosis is an area still being investigated. It has been proposed that the CTL's may release a factor possibly TNF beta that induces the cell death or through the interaction between FASL and FAS interaction between CTL and target cell through the activation of caspases.
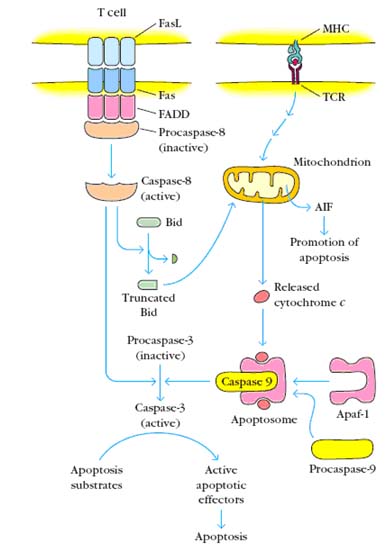
Two pathways to apoptosis occur in T cells. (a) Activated peripheral T cells are induced to express high levels of Fas and FasL. FasL induces the trimerization of Fas on a neighboring cell. FasL can also engage Fas on the same cell, resulting in a self-induced death signal. Trimerization of Fas leads to the recruitment of FADD, which leads in turn to the cleavage of associated molecules of procaspase 8 to form active caspase 8. Caspase 8 cleaves procaspase 3, producing active caspase 3, which results in the death of the cell. Caspase 8 can also cleave Bid to a truncated form that can activate the mitochondrial death pathway. (b) Other signals, such as the engagement of the TCR by peptide-MHC complexes on an APC, result in the activation of the mitochondrial death pathway. A key feature of this pathway is the release of AIF (apoptosis inducing factor) and cytochrome c from the inner mitochondrial membrane into the cytosol. Cytochrome c interacts with Apaf-1 and subsequently with procaspase 9 to form the active apoptosome. The apoptosome initiates the cleavage of procaspase 3, producing active caspase 3, which initiates the execution phase of apoptosis by proteolysis of substances whose cleavage commits the cell to apoptosis.
SUPPRESSOR T CELLS (Ts):
Another subpopulation of T cells (T suppressor cells or Ts cells) acts to inhibit TH cells from function, thereby preventing the initiation of the response. They are CD4-8+ in contrast to TH cells which are CD4+8-. Ts cells can also act directly on B cells or effector T cells to inhibit their activity, but this is probably less important than their inhibition of TH cell activity. Some autoimmune diseases are thought to result from defects in Ts function.
NULL CELLS:
A small group of peripheral blood lymphocytes, called null cells, fail to express the membrane molecules that distinguish T and B cell lineages. These cells also fail to display antigen binding receptors of either the T or B cell lineage and therefore lack the attributes of immulogic specificity and memory. There are two sub populations of null cells namely Natural Killer cells (NK cells) and Killer cells (K cells). But in most of the books null cells referred as a single population of NK cells.
NK-CELLS:
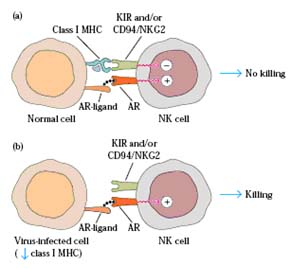
These cells are sometimes called large granular lymphocytes (LGL's). NK cells have some surface markers in common with T cells, and they are also functionally similar to cytotoxic T lymphocytes (CTL's). Like CTL's, NK cells are particularly important in the killing of cellular targets usually tumor cells. Unlike CTL's, however, the killing by NK cells is nonspecific; they do not need to recognize antigen/MHC on the target cell. NK cells do not have a T cell receptor and are not T cells. An NK cell kills a target cell by releasing perforin and other molecules which damages the target cell membrane leading to death. NK cells also cause death by inducing apoptosis in the target. The cytokine TNF alpha is released by the NK cells and may be involved in this process. They are able to recognize and kill some abnormal cells, for example some tumor cells and virus-infected cells, and are thought to be important in the innate immune defense against intracellular pathogens. The activation of NK cells basically depend upon the Killer cell Inhibitory Receptor (KIR) and Activation Receptor (AR). When both the receptors activated in the case of NK cell and normal cell interaction, there is no activation of NK cells where as activation of AR receptor alone in the case of NK cell and self altered or infected cell interaction results in the lysis of infected cell.
KILLER CELLS (K Cells):
If NK-Cells posses Fc receptor for antibodies, then they kill the infecting agents specifically. They are referred as Antibody Dependent Cytotoxic Cells (ADCC). They are then called as Killer cells.

ANTIGEN PRESENTING CELLS(APCs):
Antigen presenting cells are a functionally defined group of cells which are able to take up antigens and present them to T lymphocytes in a recognizable form in the groove of MHC molecules. Although many cells can do this the cells which are most efficient, the so-called "professional antigen presenting cells", are macrophages and dendritic cells. These are professional cells because they are highly effective at producing the "second signal" required for T cell activation. The APC first internalizes the antigen maybe in the form of a bacteria or bacterial product, processes it by breaks it down into antigenic peptides [epitopes] by digesting it with lysozyme and then expresses the antigen fragment on its surface in the groove of an MHC molecule. Now the antigen is in the form recognizable by T cells. Antigen presentation by APC is a required first step in TH cell activation. B-Cell was also posses antigen processing and presenting property due to expression of MHC-II molecule after activation.
DENDRITIC CELLS:
The dendritic cell acquired its name because it is covered with a maze of long membrane processes resembling dendrites of nerve cells. Most dendritic cells are process and present antigen to TH cells. These cells can be classified based on their location. Langerhans cells found in the epidermis and mucous membrane, Interstitial dendritic cells which populate most organs like heart, lungs, liver, kidney and gastrointestinal tract, Interdigitating dendritic cells present in T-cell areas of secondary lymphoid tissue and the thymic medulla and Circulating dendritic cells including those in the blood and those in the lymph which is otherwise known as veiled cells.
The dendritic cells in each of these locations have morphologic and functional differences. Despite their differences, all of these dendritic cells constitutively express high levels of both class II MHC molecules and the co-stimulatory B7 molecule. For this reason, they are more potent antigen presenting cells than macrophages and B cells, both of which need to be activated before they can function as APCs. After capturing antigen in the tissues by phagocytosis or by endocytosis, dendritic cells migrate into the blood or lymph and circulate to various lymphoid organs where they present the antigen to T-lymphocytes.
Follicular dendritic cells (FDCs) are cells with membranous projections present in the germinal centers of lymphoid follicles in the lymph node, spleen and mucosal lymphoid tissues. Most FDCs are not derived from precursors in the bone marrow and are unrelated to the dendritic cells that present antigens to T lymphocytes. FDCs do not express class II MHC molecules and therefore do not function as antigen presenting cells for TH cell activation. These dendritic cells were named for their exclusive location in organized structures of the lymph node, called lymph follicles, which are rich in B cells. FDCs trap antigens complexed to antibodies or complement products and display these antigens on their surfaces for recognition by B lymphocytes. This is important for the selection of activated B lymphocytes whose antigen receptors bind the displayed antigens with high affinity.
PLATELETS:
These are nonnucleated cells of about 3 micron in diameter. They are also spherical and colorless. They are produced from stem cells of bone marrow. They have low affinity receptors for Ig E and Ig G and MHC – I molecules on its surface. They play vital role in blood clotting and inflammation. The clotting itself prevents invasion of pathogens through walling off reaction. They also contain granules which secrete histamine and serotonin and their release from the platelets contribute to immediate hypersensitivity reactions.
ERYTHROCYTES (RBCs):
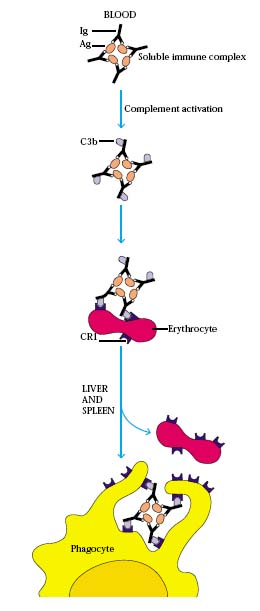
Erythrocytes plays vital role in oxygen transport but it also posses important immunological role. Erythrocytes removes antigen – antibody complex (immune complex) from circulation.
SUMMARY:
Immune responses are mediated by leukocytes, which derive from precursors in the bone marrow. A pluripotent hematopoietic stem cell gives rise to the lymphocytes responsible for adaptive immunity, and also to myeloid lineages that participate in both innate and adaptive immunity. Neutrophils, eosinophils, and basophils are collectively known as granulocytes; they circulate in the blood unless recruited to act as effector cells at sites of infection and inflammation. Macrophages and mast cells complete their differentiation in the tissues where they act as effector cells in the front line of host defense and initiate inflammation. Macrophages phagocytose bacteria, and recruit other phagocytic cells, the neutrophils, from the blood. Mast cells are exocytic and are thought to orchestrate the defense against parasites as well as triggering allergic inflammation; they recruit eosinophils and basophils, which are also exocytic. Dendritic cells enter the tissues as immature phagocytes where they specialize in ingesting antigens. These antigen presenting cells subsequently migrate into lymphoid tissue. There are two major types of lymphocyte: B lymphocytes, which mature in the bone marrow; and T lymphocytes, which mature in the thymus. The bone marrow and thymus are thus known as the central or primary lymphoid organs. Mature lymphocytes recirculate continually from the bloodstream through the peripheral or secondary lymphoid organs, returning to the bloodstream through the lymphatic vessels. Most adaptive immune responses are triggered when a recirculating T cell recognizes its specific antigen on the surface of a dendritic cell. The three major types of peripheral lymphoid tissue are the spleen, which collects antigens from the blood; the lymph nodes, which collect antigen from sites of infection in the tissues; and the mucosal-associated lymphoid tissues (MALT), which collect antigens from the epithelial surfaces of the body. Adaptive immune responses are initiated in these peripheral lymphoid tissues: T cells that encounter antigen proliferate and differentiate into antigen-specific effector cells, while B cells proliferate and differentiate into antibody-secreting cells.