Nervous and Muscular
System

Central
Nervous System: Brain
During development, the CNS forms from a long tube. As the anterior part of the
tube, which becomes the brain, folds during its continuing formation, initially
three different regions become apparent, identified as the forebrain,
midbrain, and hindbrain. These regions continue to develop, forming
subdivisions. The forebrain develops into two major subdivisions, the
cerebrum and the diencephalon. The midbrain remains as a single major
division. The hindbrain develops into three parts: the pons, medulla
oblongata, and the cerebellum. The pons, medulla oblongata, and the
midbrain are heavily interconnected and share many similar functions; for that
reason and their anatomical location, they are considered together as the
brainstem. The brain also contains four interconnected cavities, the
cerebral ventricles, which are filled with fluid and which provide support
for the brain.
Forebrain: The Cerebrum
The larger component of the forebrain, the cerebrum, consists of the right and
left cerebral hemispheres as well as some associated structures on the
underside of the brain. The cerebral hemispheres consist of the cerebral
cortex—an outer shell of gray matter composed primarily of cell
bodies that give the area a gray appearance–and an inner layer of white
matter, composed primarily of myelinated fiber tracts. The cerebral cortex
in turn overlies cell clusters, which are also gray matter and are collectively
termed the subcortical nuclei. The fiber tracts consist of the many nerve
fibers that bring information into the cerebrum, carry information out, and
connect
different areas within a hemisphere. The cortex layers of the left and right
cerebral hemispheres, although largely separated by a deep longitudinal
division, are connected by a massive bundle of nerve fibers known as the
corpus callosum.
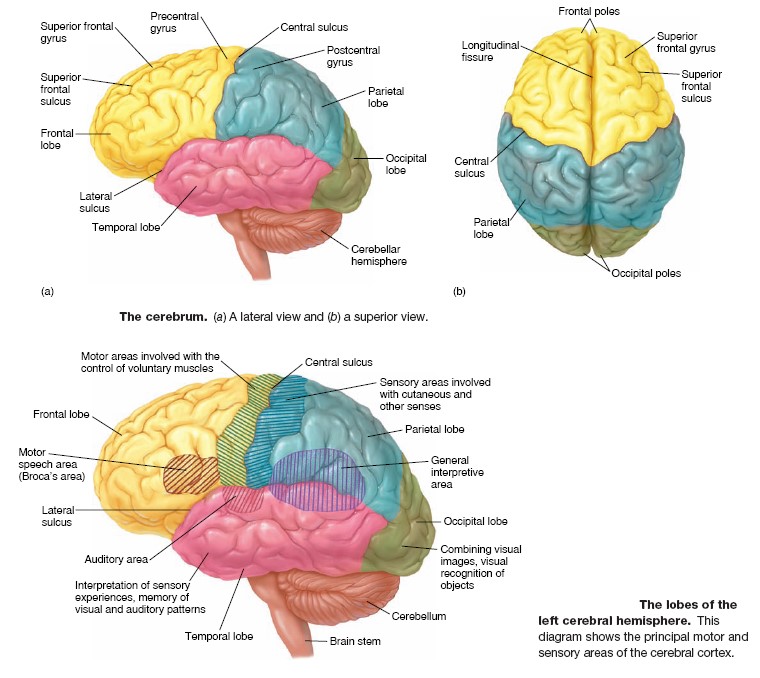
Cerebral Cortex
The
cerebral cortex of each cerebral hemisphere is divided into four lobes, named
after the overlying skull bones covering the brain: the frontal, parietal,
occipital, and temporal lobes. Although it averages only 3 mm in
thickness, the cerebral cortex is highly folded. This results in an area
containing cortical neurons that is four times larger than it would be without
folding, yet does not appreciably increase the volume of the brain. This folding
also results in the characteristic external appearance of the human cerebrum,
with its sinuous ridges called gyri (singular, gyrus) separated by
grooves called sulci (singular, sulcus). The cells of the human cerebral
cortex are organized in six distinct layers, composed of varying sizes and
numbers of two basic types: pyramidal cells (named for the shape of their cell
bodies) and nonpyramidal cells. The pyramidal cells form the major output cells
of the cerebral cortex, sending their axons to other parts of the cortex and to
other parts of the CNS. Nonpyramidal cells are mostly involved in receiving
inputs into the cerebral cortex and in local processing of information. This
elaboration of the human cerebral cortex into multiple cell layers, like its
highly folded structure, allows for an increase in the number and integration of
neurons for signal processing. Such specialization of structural surface area to
enhance function in organs throughout the body affirms the general principle of
physiology that structure and function are related. This is supported by the
fact that an increase in the number of cell layers in the cerebral cortex has
paralleled the increase in behavioral and cognitive complexity in vertebrate
evolution. For example, reptiles have just three layers in the cortex, and
dolphins have five. Some regions of the human brain with ancient evolutionary
origins, such as the olfactory cortex, persist in having only three cell layers.
The cerebral cortex is one of the most complex integrating areas of the nervous
system. It is here that basic afferent information is collected and processed
into meaningful perceptual images, and control over the systems that govern the
movement of the skeletal muscles is refined. Nerve fibers enter the cerebral
cortex predominantly from the diencephalon and areas of the brainstem; there is
also extensive signaling between areas within the cerebral cortex. Some of the
input fibers convey information about specific events in the environment,
whereas others control levels of cortical excitability, determine states of
arousal, and direct attention to specific stimuli.
Basal Nuclei
The
subcortical nuclei are heterogeneous groups of gray matters that lie deep within
the cerebral hemispheres. Predominant among them are the basal nuclei
(often, but less correctly referred to as basal ganglia), which
have an important function in controlling movement and posture and in more
complex aspects of behavior.
Limbic System
Thus
far, we have described discrete anatomical areas of the forebrain. Some of these
forebrain areas, consisting of both gray and white matter, are also classified
together in a functional system called the limbic system. This
interconnected group of brain structures includes portions of frontal-lobe
cortex, temporal lobe, thalamus, and hypothalamus, as well as the fiber pathways
that connect them. Besides being connected with each other, the parts of the
limbic system connect with many other parts of the CNS. Structures within the
limbic system are associated with learning, emotional experience and behavior,
and a wide variety of visceral and endocrine functions.
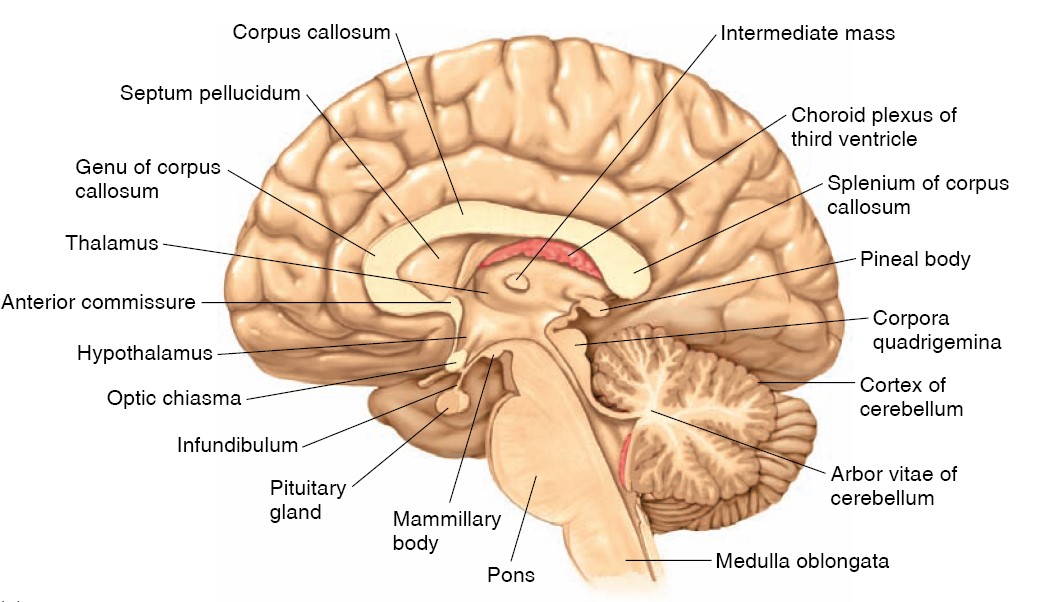
Forebrain: The Diencephalon
The diencephalon, which is divided in two by the narrow third cerebral
ventricle, is the second component of the forebrain. It contains the thalamus,
hypothalamus, and epithalamus. The thalamus is a collection of several
large nuclei that serve as synaptic relay stations and important integrating
centers for most inputs to the cortex, and it has a key function in general
arousal. The thalamus also is involved in focusing attention. For example, it is
responsible for filtering out extraneous sensory information such as might occur
when you try to concentrate on a private conversation at a loud, crowded party.
The hypothalamus lies below the thalamus and is on the undersurface of
the brain; like the thalamus, it contains numerous different nuclei. These
nuclei and their pathways form the master command center for neural and
endocrine coordination. Indeed, the hypothalamus is the single most important
control area for homeostatic regulation of the internal environment. Behaviors
having to do with preservation of the individual (for example, eating and
drinking) and preservation of the species (reproduction) are among the many
functions of the hypothalamus. The hypothalamus
lies directly above and is connected by a stalk to the pituitary gland,
an important endocrine structure that the hypothalamus regulates. As mentioned
earlier, some parts of the hypothalamus and thalamus are also considered part of
the limbic system. The
epithalamus is a small mass of tissue that includes the pineal gland,
which participates in the control of circadian rhythms through release of the
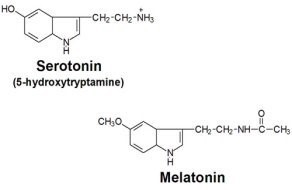
hormone melatonin.
Hindbrain: The Cerebellum
The cerebellum consists of an outer layer of cells, the cerebellar cortex (do
not confuse this with the cerebral cortex), and several deeper cell clusters.
Although the cerebellum does not initiate voluntary movements, it is an
important center for coordinating movements and for controlling posture and
balance. To carry out these functions, the cerebellum receives information from
the muscles and joints, skin, eyes, vestibular apparatus, viscera, and the parts
of the brain involved in control of movement. Although the cerebellum’s function
is almost exclusively motor, recent research strongly suggests that it also may
be involved in some forms of learning. The other components of the hindbrain—the
pons and medulla oblongata—are considered together with the midbrain.
Brainstem: The Midbrain, Pons, and Medulla Oblongata
All the nerve fibers that relay signals between the forebrain, cerebellum, and
spinal cord pass through the brainstem. Running through the core of the
brainstem and consisting of loosely arranged nuclei intermingled with bundles of
axons is the reticular formation, the one part of the brain absolutely
essential for life. It receives and integrates input from all regions of the CNS
and processes a great deal of neural information. The reticular formation is
involved in motor functions, cardiovascular and respiratory control, and the
mechanisms that regulate sleep and wakefulness and that focus attention. Most of
the biogenic amine neurotransmitters are released from the axons of cells in the
reticular formation. Because of the far-reaching projections of these cells,
these neurotransmitters affect all levels of the nervous system.
The pathways that convey information from the reticular formation to the upper
portions of the brain stimulate arousal and wakefulness. They also direct
attention to specific events by selectively stimulating neurons in some areas of
the brain while inhibiting others. The fibers that descend from the reticular
formation to the spinal cord influence activity in both efferent and afferent
neurons. Considerable interaction takes place between the reticular pathways
that go up to the forebrain, down to the spinal cord, and to the cerebellum. For
example, all three components function in controlling muscle activity.
The reticular formation encompasses a large portion of the brainstem, and many
areas within the reticular formation serve distinct functions. For example, some
reticular formation neurons are clustered together, forming brainstem nuclei and
integrating centers. These include the cardiovascular, respiratory, swallowing,
and vomiting centers. The reticular formation also has nuclei important in
eye-movement control and the reflexive orientation of the body in space. In
addition, the brainstem contains nuclei involved in processing information for
10 of the 12 pairs of cranial nerves. These are the peripheral nerves
that connect directly with the brain and innervate the muscles, glands, and
sensory receptors of the head, as well as many organs in the thoracic and
abdominal cavities.
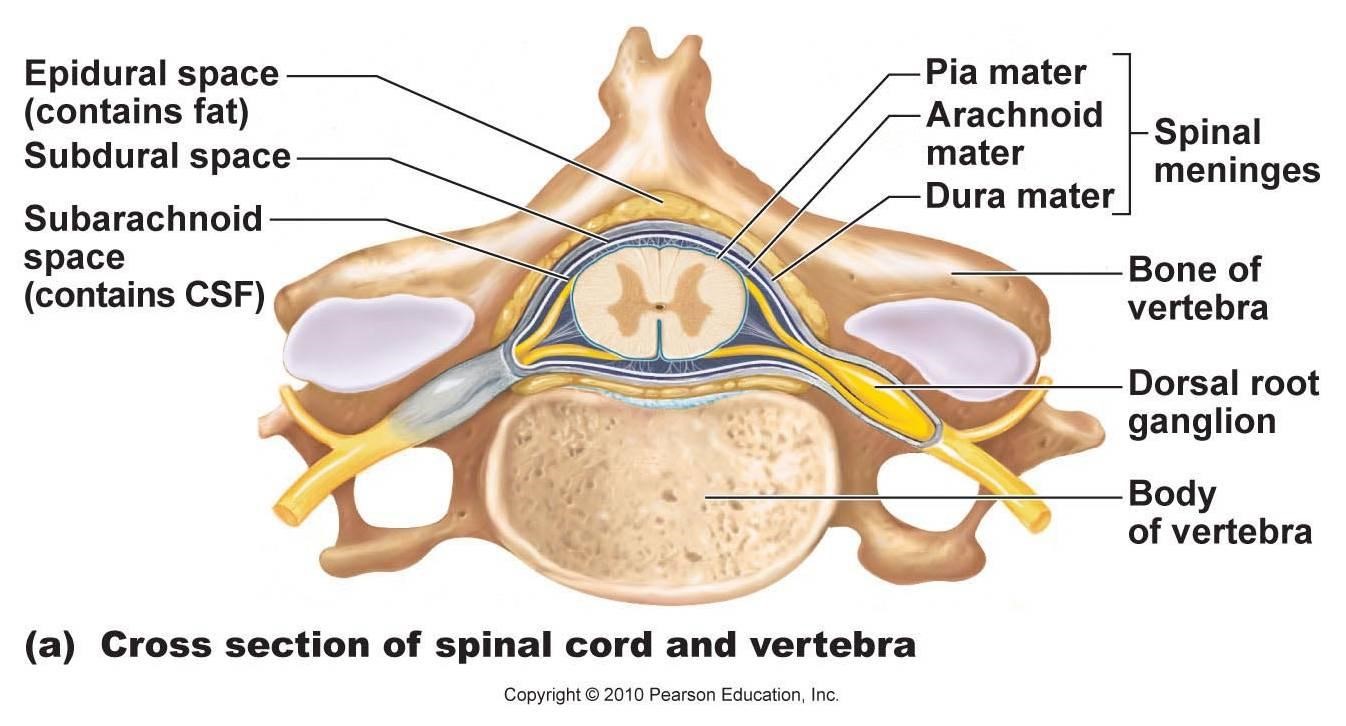
Central Nervous System: Spinal Cord
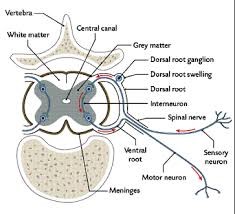
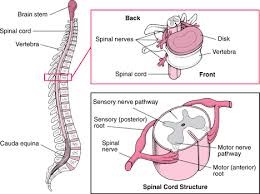
The spinal cord lies within the bony vertebral column. It is a slender cylinder
of soft tissue about as big around as your little finger. The central
butterfly-shaped area (in cross section) of gray matter is composed of
interneurons, the cell bodies and dendrites of efferent neurons, the entering
axons of afferent neurons, and glial cells. The regions of gray matter
projecting toward the back of the body are called the dorsal horns,
whereas those oriented toward the front are the ventral horns. The gray
matter is surrounded by white matter, which consists of groups of myelinated
axons. These groups of fiber tracts run longitudinally through the cord, some
descending to relay information from the brain to the spinal cord, others
ascending to transmit information to the brain. Pathways also transmit
information between different levels of the spinal cord. Groups of afferent
fibers that enter the spinal cord from the peripheral nerves enter on the dorsal
side of the cord via the dorsal roots. Small bumps on the dorsal roots,
the dorsal root ganglia, contain the cell bodies of these afferent
neurons. The axons of
efferent neurons leave the spinal cord on the ventral side via the ventral
roots. A short distance from the cord, the dorsal and ventral roots from the
same level combine to form a spinal nerve, one on each side of the spinal
cord, carrying two-way information from afferents and efferents.
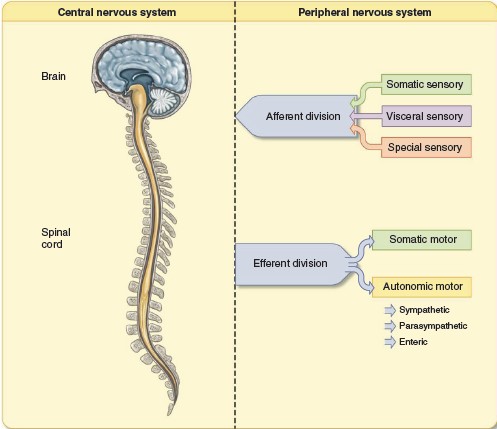
Peripheral Nervous System
Neurons in the PNS transmit signals between the CNS and receptors and effectors
in all other parts of the body. As noted earlier, the axons are grouped into
bundles called nerves. The PNS has 43 pairs of nerves: 12 pairs of cranial
nerves and 31 pairs of spinal nerves that connect with the spinal cord. The 31
pairs of spinal nerves are designated by the vertebral levels from which they
exit: cervical, thoracic, lumbar, sacral, and coccygeal. Neurons in the spinal
nerves at each level generally communicate with nearby structures, controlling
muscles and glands as well as receiving
sensory input. The
eight pairs of cervical nerves innervate the
neck, shoulders, arms, and hands. The 12 pairs of thoracic nerves are associated
with the chest and upper abdomen. The five pairs of lumbar nerves are associated
with the lower abdomen, hips, and legs; the five pairs of sacral nerves are
associated with the genitals and lower digestive tract. A single pair of
coccygeal nerves associated with the skin over the region of the tailbone brings
the total to 31 pairs. These peripheral nerves can contain nerve fibers that are
the axons of efferent neurons, afferent neurons, or both.
Therefore, fibers in a nerve may be classified as belonging to the
efferent or the afferent division of the PNS. All the spinal nerves
contain both afferent and efferent fibers, whereas some of the cranial nerves
contain only afferent fibers (the optic nerves from the eyes, for example) or
only efferent fibers (the hypoglossal nerve to muscles of the tongue, for
example). As noted earlier, afferent neurons convey information from sensory
receptors at their peripheral endings to the CNS. The long part of their axon is
outside the CNS and is part of the PNS. Afferent neurons are sometimes called
primary afferents or firstorder neurons because they are the first cells
entering the CNS in the synaptically linked chains of neurons that handle
incoming information. Efferent
neurons carry signals out from the CNS to muscles, glands, and other tissues.
The efferent division of the PNS is more complicated than the afferent, being
subdivided into a somatic nervous system and an autonomic nervous
system. These terms are somewhat misleading because they suggest the
presence of additional nervous systems distinct from the central and peripheral
systems. Keep in mind that these terms together make up the efferent division of
the PNS.
The simplest distinction between the somatic and autonomic systems is that the
neurons of the somatic division innervate skeletal muscle, whereas the autonomic
neurons innervate smooth and cardiac muscle, glands, neurons in the
gastrointestinal tract, and other tissues. The
somatic portion of the efferent division of the PNS is made up of all the nerve
fibers going from the CNS to skeletal muscle cells. The cell bodies of these
neurons are located in groups in the brainstem or the ventral horn of the spinal
cord. Their large-diameter, myelinated axons leave the CNS and pass without any
synapses to skeletal muscle cells. The neurotransmitter these neurons release is
acetylcholine. Because activity in the somatic neurons leads to contraction of
the innervated skeletal muscle cells, these neurons are called motor neurons.
Excitation of motor neurons leads only to the contraction of skeletal
muscle cells; there are no somatic neurons that inhibit skeletal muscles. Muscle
relaxation involves the inhibition of the motor neurons in the spinal cord.
Cranial nerves
|
Name
|
Fibers
|
Comments
|
|
I. Olfactory |
Afferent |
Carries input from receptors in olfactory (smell) neuroepithelium* |
|
II. Optic |
Afferent |
Carries input from receptors in eye* |
|
III. Oculomotor |
Efferent Afferent |
Innervates skeletal muscles that move eyeball up, down, and medially,
and raise upper eyelid; innervates smooth muscles that constrict pupil
and alter lens shape for near and far vision Transmits information from
receptors in muscles |
|
IV. Trochlear |
Efferent |
Innervates skeletal muscles that move eyeball downward and laterally |
|
|
Afferent |
Transmits information from receptors in muscles |
|
V. Trigeminal |
Efferent |
Innervates skeletal chewing muscles |
|
|
Afferent |
Transmits information from receptors in skin; skeletal muscles of face,
nose, and mouth; and teeth sockets |
|
VI. Abducens |
Efferent |
Innervates skeletal muscles that move eyeball laterally |
|
|
Afferent |
Transmits information from receptors in muscles |
|
VII. Facial |
Efferent Afferent |
Innervates skeletal muscles of facial expression and swallowing;
innervates nose, palate, and lacrimal and salivary glands Transmits
information from taste buds in front of tongue and mouth |
|
VIII. Vestibulocochlear |
Afferent |
Transmits information from receptors in inner ear |
|
IX. Glossopharyngeal |
Efferent Afferent |
Innervates skeletal muscles involved in swallowing and parotid salivary
gland Transmits information from taste buds at back of tongue and
receptors in auditory-tube skin; also transmits information from carotid
artery baroreceptors (blood pressure receptors) and from chemoreceptors
that detect changes in blood gas levels |
|
X. Vagus |
Efferent Afferent |
Innervates skeletal muscles of pharynx and larynx and smooth muscle and
glands of thorax and abdomen Transmits information from receptors in
thorax and abdomen |
|
XI. Accessory |
Efferent |
Innervates sternocleidomastoid and trapezius muscles in the neck |
|
XII. Hypoglossal |
Efferent |
Innervates skeletal muscles of tongue |
|
*The olfactory and optic pathways are CNS structures so are not
technically “nerves.” |
||
THE SYNAPSE
Axons end close to, or in some cases at the point of contact with, another cell.
Once action potentials reach the end of an axon, they directly or indirectly
stimulate (or inhibit) the other cell. In specialized cases, action potentials
can directly pass from one cell to another. In most
cases, however, the action potentials stop at the axon terminal, where they
stimulate the release of a chemical neurotransmitter that affects the next cell.
A synapse is the functional connection between a neuron and a second
cell. In the CNS, this other cell is also a neuron. In the PNS, the other cell
may be either a neuron or an effector cell within a muscle or gland.
Although the physiology of neuron-neuron synapses and neuron-muscle synapses is
similar, the latter synapses are often called myoneural, or
neuromuscular, junctions.
Neuron-neuron synapses usually involve a connection between the axon of one
neuron and the dendrites, cell body, or axon of a second neuron. These are
called, respectively, axodendritic, axosomatic, and axoaxonic
synapses. In almost all synapses, transmission is in one direction only—from
the axon of the first (or presynaptic) neuron to the second (or
postsynaptic) neuron. Most commonly, the synapse occurs between the axon of
the presynaptic neuron and the dendrites or cell body of the postsynaptic
neuron. In the early part of the twentieth century, most physiologists believed
that synaptic transmission was electrical —that is, that action
potentials were conducted directly from one cell to the next. This was a logical
assumption, given that nerve endings appeared to touch the postsynaptic cells
and that the delay in synaptic conduction was extremely short (about 0.5 msec).
Improved histological techniques, however, revealed tiny gaps in the synapses,
and experiments demonstrated that the actions of autonomic nerves could be
duplicated by certain chemicals. This led to the
hypothesis that synaptic transmission might be chemical —that
the presynaptic nerve endings might release chemicals called
neurotransmitters that stimulated action potentials in the postsynaptic
cells.
In 1921 a physiologist named Otto Loewi
published the results of experiments suggesting that synaptic transmission was
indeed chemical, at least at the junction between a branch of the vagus nerve
and the heart. He had isolated the heart of a frog
and, while stimulating the branch of the vagus that innervates the heart,
perfused the heart with an isotonic salt solution. Stimulation of the vagus
nerve was known to slow the heart rate. After stimulating the vagus nerve to
this frog heart, Loewi collected the isotonic salt solution and then gave it to
a second heart. The vagus nerve to this second heart was not stimulated, but the
isotonic solution from the first heart caused the second heart to also slow its
beat. Loewi concluded that the nerve endings of the vagus must have released a
chemical—which he called Vagusstoff
- that inhibited the heart rate. This chemical was subsequently identified
as acetylcholine, or ACh. In the decades following Loewi’s
discovery, many other examples of chemical synapses were discovered, and the
theory of electrical synaptic transmission fell into disrepute. More recent
evidence, ironically, has shown that electrical synapses do exist in the nervous
system (though they are the exception), within smooth muscles, and between
cardiac cells in the heart.
Electrical Synapses: Gap Junctions
In order for two cells to be electrically coupled, they must be approximately
equal in size and they must be joined by areas of contact with low electrical
resistance. In this way, impulses can be regenerated from one cell to the next
without interruption. Adjacent cells that are electrically coupled are joined
together by gap junctions. In gap junctions, the membranes of the two
cells are separated by only 2 nanometers (1 nano meter = 10 − 9 meter). A
surface view of gap junctions in the electron microscope reveals hexagonal
arrays of particles that function as channels through which ions and molecules
may pass from one cell to the next. Each gap junction is now known to be
composed of 12 proteins known as connexins, which are arranged like
staves of a barrel to form a water-filled pore.
Gap junctions are present in cardiac muscle, where they allow action potentials
to spread from cell to cell, so that the myocardium can contract as a unit.
Similarly, gap junctions in some smooth muscles allow many cells to be
stimulated and contract together, producing a stronger contraction (as in the
uterus during labor). The function of gap junctions in the nervous system is
less well understood; nevertheless, gap junctions are found between neurons in
the brain, where they can synchronize the firing of groups of neurons. Gap
junctions are also found between neuroglial cells, where they are believed to
allow the passage of Ca 2 + and perhaps other ions and molecules between the
connected cells. The function of gap junctions is more complex than was once
thought. Neurotransmitters and other stimuli, acting through second messengers
such as cAMP or Ca 2 +, can lead to the phosphorylation or dephosphorylation of
gap junction connexin proteins, causing the opening or closing of gap junction
channels. For example, light causes the ion conductance through the gap
junctions between neurons in the retina to increase in some neurons and decrease
in others.
Chemical Synapses
Transmission across the majority of synapses in the nervous system is one-way
and occurs through the release of chemical neurotransmitters from presynaptic
axon endings. These presynaptic endings, called terminal boutons (from
the Middle French bouton = button) because of their swollen appearance,
are separated from the postsynaptic cell by a synaptic cleft so narrow
(about 10 nm) that it can be seen clearly only with an electron microscope.
Chemical transmission requires that the synaptic cleft stay very narrow and that
neurotransmitter molecules are released near their receptor proteins in the
postsynaptic membrane. The physical association of the pre- and postsynaptic
membranes at the chemical synapse is stabilized by the action of particular
membrane proteins. Cell adhesion molecules (CAMs) are proteins in the
pre- and postsynaptic membranes that project from these membranes into the
synaptic cleft, where they bond to each other. This Velcro-like effect ensures
that the pre- and postsynaptic membranes stay in close proximity for rapid
chemical transmission.
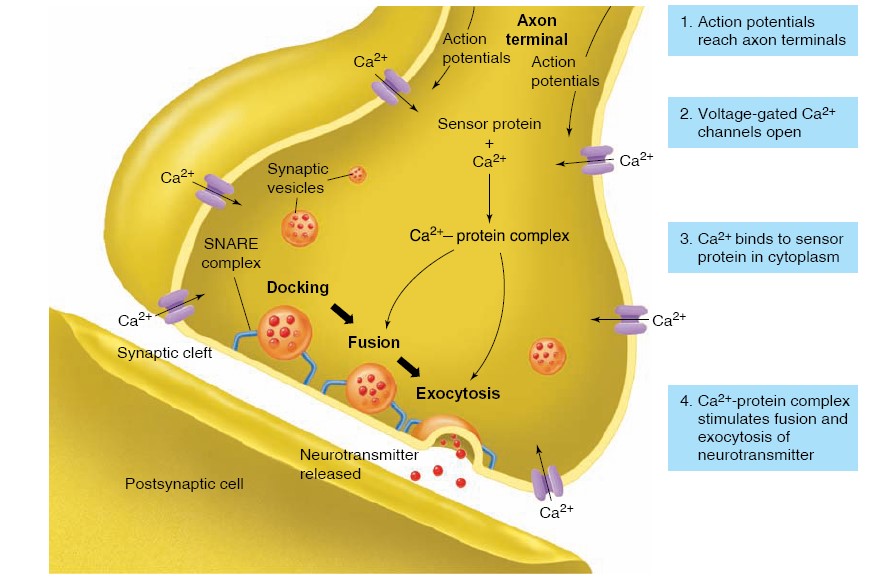
Release of Neurotransmitter
Neurotransmitter molecules within the presynaptic neuron endings are contained
within many small, membrane-enclosed synaptic vesicles. In order for the
neurotransmitter within these vesicles to be released into the synaptic cleft,
the vesicle membrane must fuse with the axon membrane in the process of
exocytosis. Exocytosis of synaptic vesicles, and the consequent release of
neurotransmitter molecules into the synaptic cleft, is triggered by action
potentials that stimulate the entry of Ca2+ into the axon terminal
through voltage-gated Ca2+ channels. When there is a greater
frequency of action potentials at the axon terminal, there is a greater entry of
Ca2+, and thus a larger number of synaptic vesicles undergoing
exocytosis and releasing neurotransmitter molecules. As a result, a greater
frequency of action potentials by the presynaptic axon will result in greater
stimulation of the postsynaptic neuron. Ca2+ entering the axon
terminal binds to a protein, believed to be synaptotagmin, which serves
as a Ca2+ sensor, forming a Ca 2 + -synaptotagmin complex in the
cytoplasm. This occurs close to the location where synaptic vesicles are already
docked (attached) to the plasma membrane of the axon terminal. At this
stage, the docked vesicles are bound to the plasma membrane of the presynaptic
axon by complexes of three SNARE proteins that bridge the vesicles and
plasma membrane. The complete fusion of the vesicle membrane and plasma
membrane, and the formation of a pore that allows the release of
neurotransmitter, occurs when the Ca2+ -synaptotagmin complex
displaces a component of the SNARE, or fusion, complex.
This process is very rapid: exocytosis of neurotransmitter occurs less than 100
microseconds after the intracellular Ca 2 + concentration rises (SNARE- Soluble
NSF Attachment protein Receptor; NSF – N-ethyl maleimide Sensitive Factor).
Action of Neurotransmitter
Once the neurotransmitter molecules have been released from the presynaptic axon
terminals, they diffuse rapidly across the synaptic cleft and reach the membrane
of the postsynaptic cell. The neurotransmitters then bind to specific
receptor proteins that are part of the postsynaptic membrane. Receptor
proteins have high specificity for their neurotransmitter, which is the
ligand of the receptor protein. The term ligand in this case refers
to a smaller molecule (the neurotransmitter) that binds to and forms a complex
with a larger protein molecule (the receptor). Binding of the neurotransmitter
ligand to its receptor protein causes ion channels to open in the postsynaptic
membrane. The gates that regulate these channels, therefore, can be called
chemically regulated (or ligand-regulated) gates because they
open in response to the binding of a chemical ligand to its receptor in the
postsynaptic plasma membrane.
NEURONS AND SUPPORTING CELLS
The nervous system is composed of neurons, which produce and conduct
electrochemical impulses, and supporting cells, which assist the functions of
neurons. Neurons are classified functionally and structurally; the various types
of supporting cells perform specialized functions.
Neurons
Although neurons vary considerably in size and shape, they generally have three
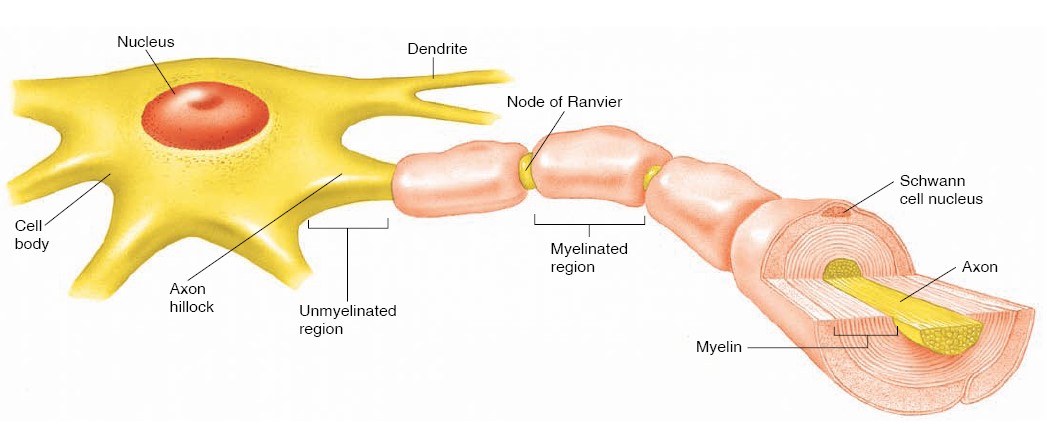
principal regions: (1) a cell body, (2) dendrites, and (3) an axon. Dendrites
and axons can be referred to generically as processes, or extensions from
the cell body. The cell body
is the enlarged portion of the neuron that contains the nucleus. It is the
“nutritional center” of the neuron where macromolecules are produced. The cell
body and larger dendrites (but not axons) contain Nissl bodies, which are
seen as dark-staining granules under the microscope. Nissl bodies are composed
of large stacks of rough endoplasmic reticulum that are needed for the synthesis
of membrane proteins. The cell bodies within the CNS are frequently clustered
into groups called nuclei (not to be confused with the nucleus of a
cell). Cell bodies in the PNS usually occur in clusters called ganglia.
Dendrites
(from the Greek dendron = tree branch) are thin, branched processes that
extend from the cytoplasm of the cell body. Dendrites provide a receptive area
that transmits graded electrochemical impulses to the cell body. The axon
is a longer process that conducts impulses, called action potentials,
away from the cell body. Axons vary in length from only a millimeter long to up
to a meter or more (for those that extend from the CNS to the foot). The origin
of the axon near the cell body is an expanded region called the axon hillock;
it is here that action potentials originate. Side branches called axon
collaterals may extend from the axon. Because axons can be quite long,
special mechanisms are required to transport organelles and proteins from the
cell body to the axon terminals. This axonal transport is
energy-dependent and is often divided into a fast component and two
slow components. The fast component (at 200 to 400 mm/day) mainly transports
membranous vesicles. One slow component (at 0.2 to 1 mm/day) transports
microfilaments and microtubules of the cytoskeleton, while the other slow
component (at 2 to 8 mm/day) transports over 200 different proteins, including
those critical for synaptic function. The slow components appear to transport
their cargo in fast bursts with frequent pauses, so that the overall rate of
transport is much slower than that occurring in the fast component.
Axonal transport may occur from the cell body to the axon and dendrites.
This direction is called anterograde transport, and involves molecular
motors of kinesin proteins that move cargo along the microtubules of the
cytoskeleton. For example, kinesin motors move synaptic vesicles, mitochondria,
and ion channels from the cell body through the axon. Similar anterograde
transport occurs in the dendrites, as kinesin moves postsynaptic receptors for
neurotransmitters and ion channels along the microtubules in the dendrites.
By contrast, axonal transport in the opposite direction-that is, along the axon
and dendrites toward the cell body-is known as retrograde transport and
involves molecular motor proteins of dyneins. The dyneins move membranes,
vesicles, and various molecules along microtubules of the cytoskeleton toward
the cell body of the neuron. Retrograde transport can also be responsible for
movement of herpes virus, rabies virus, and tetanus toxin from the nerve
terminals into cell bodies.
Classification of Neurons and Nerves
Neurons may be classified according to their function or structure. The
functional classification is based on the direction in which they conduct
impulses. Sensory, or afferent, neurons conduct impulses from
sensory receptors into the CNS. Motor, or efferent, neurons
conduct impulses out of the CNS to effector organs (muscles and glands).
Association neurons, or interneurons, are located entirely within
the CNS and serve the associative, or integrative, functions of the nervous
system. There are two types of motor neurons: somatic and autonomic. Somatic
motor neurons are responsible for both reflex and voluntary control of
skeletal muscles. Autonomic motor neurons innervate (send axons to) the
involuntary effectors—smooth muscle, cardiac muscle, and glands. The cell bodies
of the autonomic neurons that innervate these organs are located outside the CNS
in autonomic ganglia. There are two subdivisions of autonomic neurons:
sympathetic and parasympathetic. Autonomic motor neurons, together
with their central control centers, constitute the autonomic nervous system.
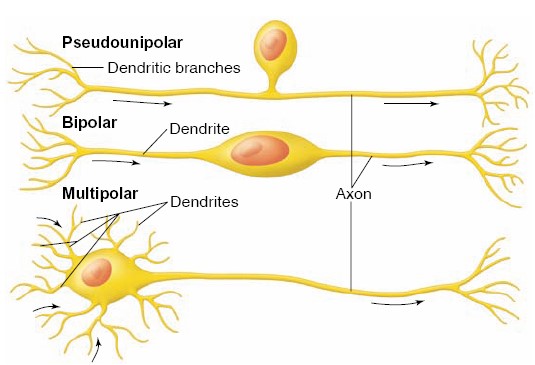
The structural classification of neurons is based on the number of processes
that extend from the cell body of the neuron. Pseudounipolar neurons have
a single short process that branches like a T to form a pair of longer
processes. They are called pseudounipolar (from the Late Latin pseudo =
false) because, although they originate with two processes, during early
embryonic development their two processes converge and partially fuse. Sensory
neurons are pseudounipolar—one of the branched processes receives sensory
stimuli and produces nerve impulses; the other delivers these impulses to
synapses within the brain or spinal cord. Anatomically, the part of the process
that conducts impulses toward the cell body can be considered a dendrite, and
the part that conducts impulses away from the cell body can be considered an
axon. Functionally, however, the branched process behaves as a single, long axon
that continuously conducts action potentials (nerve impulses). Only the small
projections at the receptive end of the process function as typical dendrites,
conducting graded electrochemical impulses rather than action potentials.
Bipolar neurons have two processes, one at either end; this type is found in
the retina of the eye. Multipolar neurons, the most common type, have
several dendrites and one axon extending from the cell body; motor neurons are
good examples of this type.
A nerve is a bundle of axons located outside the CNS. Most nerves are
composed of both motor and sensory fibers and are thus called mixed nerves.
Some of the cranial nerves, however, contain sensory fibers only. These are
the nerves that serve the special senses of sight, hearing, taste, and smell. A
bundle of axons in the CNS is called a tract.
Supporting Cells
Unlike other organs that are “packaged” in connective tissue derived from
mesoderm (the middle layer of embryonic tissue), most of the supporting cells of
the nervous system are derived from the same embryonic tissue layer (ectoderm)
that produces neurons. The term neuroglia (or glia) traditionally
refers to the supporting cells of the CNS, but in current usage the supporting
cells of the PNS are often also called glial cells.
There are two types of supporting cells in the peripheral nervous system:
1. Schwann cells
(also called neurolemmocytes), which form myelin sheaths
around peripheral axons; and
2. Satellite cells,
or ganglionic gliocytes, which support neuron cell bodies
within the ganglia of the PNS.
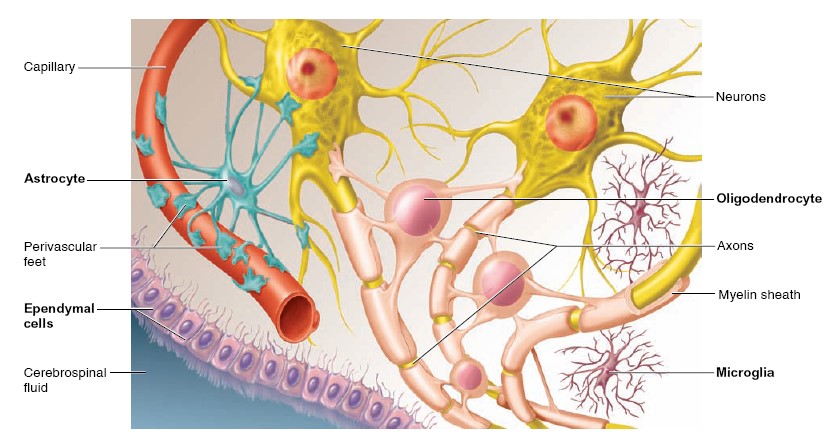
There are four types of supporting cells in the central nervous system:
1. Oligodendrocytes,
which form myelin sheaths around axons of the CNS;
2. Microglia,
which migrate through the CNS and phagocytose foreign and
degenerated material;
3. Astrocytes,
which help to regulate the external environment of neurons in the
CNS; and
4. Ependymal cells,
which line the ventricles (cavities) of the brain and the central
canal of the spinal cord.
Microglia are of hematopoietic (bone marrow) origin, and indeed can be
replenished by monocytes (a type of leukocyte)
from the blood. They
remove toxic debris within the brain and secrete anti-inflammatory factors,
functions that are essential for the health of neurons. Yet their actions have a
negative side; overactive microglial cells can release free radicals that
promote oxidative stress. and thereby contribute to neurodegenerative diseases.
Neurotransmitters and Neuromodulators
Neurons are often referred to using the suffix -ergic; the missing prefix
is the type of neurotransmitter the neuron releases. For example,
dopaminergic applies to neurons that release the neurotransmitter dopamine
(EPSPs – Excitatory Post Synaptic Potentials or IPSPs – Inhibitory Post Synaptic
Potentials). Neurotransmitters are the agents which are responsible for nerve
impulse transmission.
Neuromodulators are the agents which are responsible for complex role as
neurotransmitter, paracrine factor, and hormone etc.. They are acting both in
sympathetic and parasympathetic neurons.
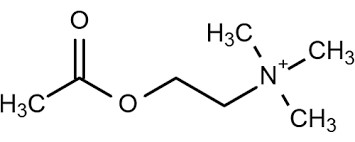
Acetylcholine
Acetylcholine
(ACh) is a major neurotransmitter in the PNS at the
neuromuscular junction and in the brain. Neurons that release ACh are called
cholinergic neurons. The cell bodies of the brain’s cholinergic neurons are
concentrated in relatively few areas, but their axons are widely distributed.
Acetylcholine is synthesized from choline (a common nutrient found in many
foods) and acetyl coenzyme A in the cytoplasm of synaptic terminals and stored
in synaptic vesicles. After it is released and activates receptors on the
postsynaptic membrane, the concentration of ACh at the postsynaptic membrane
decreases (thereby stopping receptor activation) due to the action of the enzyme
acetylcholinesterase. This enzyme is located on the presynaptic and
postsynaptic membranes and rapidly destroys ACh, releasing choline and acetate.
The choline is then transported back into the presynaptic axon terminals where
it is reused in the synthesis of new ACh. Some chemical weapons, such as the
nerve gas Sarin, inhibit acetylcholinesterase, causing a buildup
of ACh in the synaptic cleft. This results in overstimulation of postsynaptic
ACh receptors, initially causing uncontrolled muscle contractions but ultimately
leading to receptor desensitization and paralysis.
There are two general types of ACh receptors, and they are distinguished by
their responsiveness to two different chemicals.
Nicotinic
Acetylcholine Receptors
Recall
that although a receptor is considered specific for a given ligand, such as ACh,
most receptors will recognize natural or synthetic compounds that exhibit some
degree of chemical similarity to that ligand. Some ACh receptors respond not
only to acetylcholine but to the compound nicotine and have therefore come to be
known as nicotinic receptors. Nicotine is a plant alkaloid
compound that constitutes 1% to 2% of tobacco products. It is also contained in
treatments for smoking cessation, such as nasal sprays, chewing gums, and
transdermal patches. Nicotine’s hydrophobic structure allows rapid absorption
through lung capillaries, mucous membranes, skin, and the blood–brain barrier.
The nicotinic acetylcholine receptor is an excellent example of a receptor that
contains an ion channel (i.e., a ligand-gated ion channel). In this case, the
channel is permeable to both sodium and potassium ions, but because Na+
has the larger electrochemical driving force, the net effect of opening these
channels is depolarization. Nicotinic receptors are present at the neuromuscular
junction and, several nicotinic
receptor antagonists are toxins that induce paralysis. Nicotinic receptors in
the brain are important in cognitive functions and behavior. For example, one
cholinergic system that employs nicotinic receptors has a major function in
attention, learning, and memory by reinforcing the ability to detect and respond
to meaningful stimuli. The presence of nicotinic receptors on presynaptic
terminals in reward pathways of the brain explains why tobacco products are
among the most highly addictive substances known.
Muscarinic Acetylcholine Receptors
The other general type of cholinergic receptor is stimulated not only by
acetylcholine but by muscarine, a poison contained in some mushrooms; therefore,
these are called muscarinic receptors.
These receptors are
metabotropic and couple with G proteins, which then alter the activity of a
number of different enzymes and ion channels. They are prevalent at some
cholinergic synapses in the brain and at junctions where a major division of the
PNS innervates peripheral glands, tissues, and organs, like salivary glands,
smooth muscle cells, and the heart. Atropine is a naturally
occuring antagonist of muscarinic receptors with many clinical uses, such as in
eyedrops that relax the smooth muscles of the iris, thereby dilating the pupils
for an eye exam.
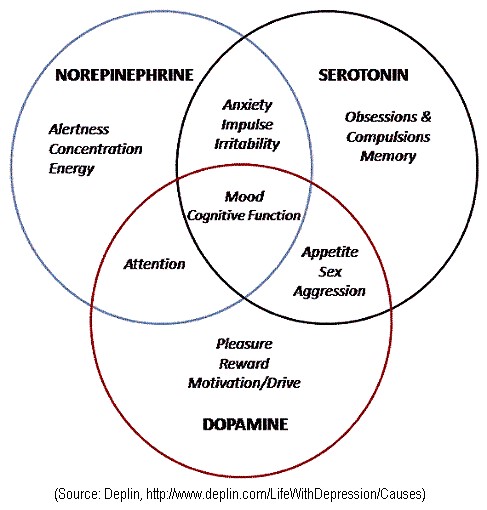
Biogenic Amines
The biogenic amines are small, charged molecules that are synthesized
from amino acids and contain an amino group (R}NH2). The most common biogenic
amines are dopamine, norepinephrine, serotonin, and histamine. Epinephrine,
another biogenic amine, is not a common neurotransmitter in the CNS but is the
major hormone secreted by the adrenal medulla. Norepinephrine is an
important neurotransmitter in both the central and peripheral components of the
nervous system.
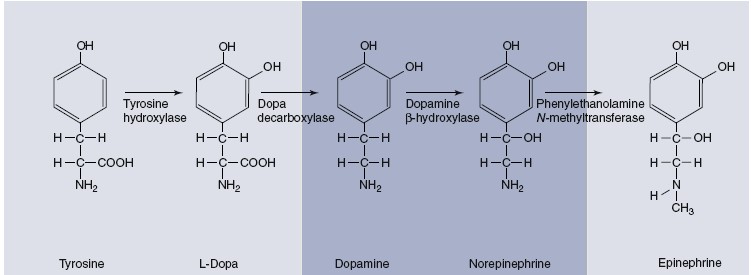
Catecholamines
Dopamine (DA), norepinephrine
(NE), and epinephrine all contain a catechol
ring (a six-carbon ring with two adjacent hydroxyl groups) and an amine group,
which is why they are called catecholamines. The catecholamines are
formed from the amino acid tyrosine and share the same two initial steps in
their synthetic pathway. Synthesis of catecholamines begins with the uptake of
tyrosine by the axon terminals and its conversion to another precursor,
L-dihydroxy-phenylalanine (L-dopa) by the rate-limiting enzyme in the
pathway, tyrosine hydroxylase. Depending on the enzymes expressed in a given
neuron, any one of the three catecholamines may ultimately be released.
Autoreceptors on the presynaptic terminals strongly modulate synthesis and
release of the catecholamines. After activation of the receptors on the
postsynaptic cell, the catecholamine concentration in the synaptic cleft
declines, mainly because a membrane transporter protein actively transports the
catecholamine back into the axon terminal. The catecholamine neurotransmitters
are also broken down in both the extracellular fluid and the axon terminal by
enzymes such as monoamine oxidase (MAO). Drugs known as
monoamine oxidase (MAO) inhibitors increase the amount of
norepinephrine and dopamine in a synapse by slowing their metabolic degradation.
Among other things, they are used in the treatment of mood disorders such as
some types of depression.
Within the CNS, the cell bodies of the catecholamine releasing neurons lie in
the brainstem and hypothalamus. Although these neurons are relatively few in
number, their axons branch greatly and go to virtually all parts of the brain
and spinal cord. These neurotransmitters have essential functions in states of
consciousness, mood, motivation, directed attention, movement, blood pressure
regulation, and hormone release. Epinephrine and norepinephrine are also
synthesized in the adrenal glands. For historical reasons having to do with
nineteenth-century physiologists referring to secretions of the adrenal gland as
“adrenaline,” the adjective “adrenergic” is commonly used to describe
neurons that release norepinephrine
or epinephrine and also to describe the receptors to which those
neurotransmitters bind. There are two major classes of receptors for
norepinephrine and epinephrine: alpha-adrenergic receptors
(alpha-adrenoceptors) and beta-adrenergic receptors (betaadrenoceptors).
All catecholamine receptors are metabotropic, and thus use second messengers to
transfer a signal from the surface of the cell to the cytoplasm.
Alpha-adrenoceptors exist in two subclasses, a1 and a2. They act presynaptically
to inhibit
norepinephrine release (a2) or postsynaptically to either stimulate or inhibit
the activity of different types of K+ channels (a1). Betaadrenoceptors act via stimulatory G proteins to
increase cAMP in the postsynaptic cell. There are three subclasses of
beta-receptors, b1, b2, and b3, which function in different ways in different
tissues. The subclasses of alpha- and beta-receptors are distinguished by the
drugs that influence them and their second-messenger systems.
Serotonin:
Serotonin
(5-hydroxytryptamine, or 5-HT) is produced from tryptophan, an
essential amino acid. Its effects generally have a slow onset, indicating that
it works as a neuromodulator. Serotonergic neurons innervate virtually every
structure in the brain and spinal cord and operate via at least 16 different
receptor subtypes. In general, serotonin has an excitatory effect on pathways
that are involved in the control of muscles, and an inhibitory effect on
pathways that mediate sensations. The activity of serotonergic neurons is lowest
or absent during sleep and highest during states of alert wakefulness. In
addition to their contributions to motor activity and sleep, serotonergic
pathways also function in the regulation of food intake, reproductive behavior,
and emotional states such as mood and anxiety.
Selective serotonin reuptake inhibitors such as paroxetine (Paxil)
are thought to aid in the treatment of depression by inactivating the
presynaptic membrane 5-HT transporter, which mediates the reuptake of serotonin
into the presynaptic cell. This, in turn, increases the synaptic concentration
of the neurotransmitter. Interestingly, such drugs are often associated with
decreased appetite but paradoxically cause weight gain due to disruption of
enzymatic pathways that regulate fuel metabolism. This is one example of how the
use of reuptake inhibitors for a specific neurotransmitter—one with widespread
actions—can cause unwanted side effects. Serotonin is found in both neural and
nonneural cells, with the majority located outside of the CNS. In fact,
approximately 90% of the body’s total serotonin is found in the digestive
system, 8% is in blood platelets and immune cells, and only 1% to 2% is found in
the brain. The drug lysergic acid diethylamide (LSD) stimulates
the 5-HT2A subtype of serotonin receptor in the brain. Though the mechanism is
not completely understood, alteration of this receptor complex produces the
intense visual hallucinations that are produced by ingestion of LSD.
Amino Acid Neurotransmitters
In addition to the neurotransmitters that are synthesized from amino acids,
several amino acids themselves function as neurotransmitters. Although the amino
acid neurotransmitters chemically fit the category of biogenic amines, they are
traditionally placed into a category of their own. The amino acid
neurotransmitters are by far the most prevalent neurotransmitters in the CNS,
and they affect virtually all neurons there.
Glutamate
There
are a number of excitatory amino acids, but the most common by far is
glutamate, which is estimated to be the primary neurotransmitter at 50% of
excitatory synapses in the CNS. As with other neurotransmitters, pharmacological
manipulation of the receptors for glutamate has permitted identification of
specific receptor subtypes by their ability to bind natural and synthetic
ligands. Although metabotropic glutamate receptors do exist, the vast majority
are ionotropic, with two important subtypes being found in postsynaptic
membranes. They are designated as AMPA receptors (identified by their
binding to a-amino-3 hydroxy-5 methyl-4 isoxazole propionic acid) and NMDA
receptors (which bind N-methyl-D-aspartate). Cooperative activity of
AMPA and NMDA receptors has been implicated in one type of a phenomenon called
longterm potentiation (LTP). This mechanism couples frequent
activity across a synapse with lasting changes in the strength of signaling
across that synapse and is thus thought to be one of the major cellular
processes involved in learning and memory.
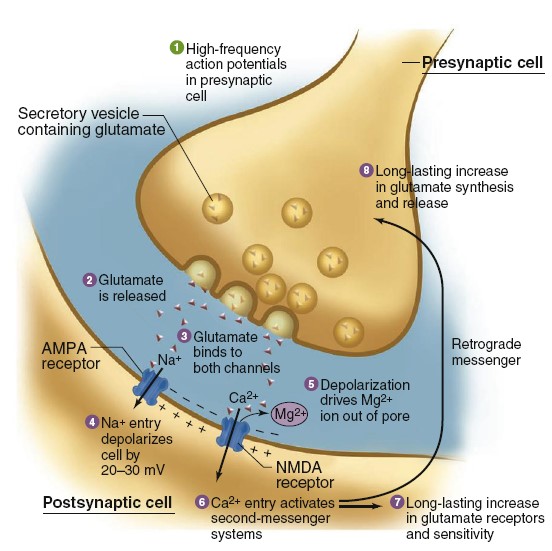
When a presynaptic neuron fires action potentials (step 1), glutamate is
released from presynaptic terminals (step 2) and binds to both AMPA and NMDA
receptors on postsynaptic membranes (step 3). AMPA receptors function just like
the excitatory postsynaptic receptors discussed earlier—when glutamate binds,
the channel becomes permeable to both Na+ and K+, but the
larger entry of Na+ creates a depolarizing EPSP of the postsynaptic
cell (step 4). By contrast, NMDA-receptor channels also mediate a substantial Ca+
flux, but opening them requires more than just glutamate binding. A magnesium
ion blocks NMDA channels when the membrane voltage is near the negative resting
potential, and to drive it out of the way the membrane must be significantly
depolarized by the current through AMPA channels (step 5). This explains why it
requires a high frequency of presynaptic action potentials to complete the
longterm potentiation mechanism. At low frequencies, there is insufficient
temporal summation of AMPA-receptor EPSPs to provide the 20–30 mV of
depolarization needed to move the magnesium ion, and so the NMDA receptors do
not open. When the depolarization is sufficient, however, NMDA receptors do
open, allowing Ca2+ to enter the postsynaptic cell (step 6). Calcium
ions then activate a second-messenger cascade in the postsynaptic cell that
includes persistent activation of multiple different protein kinases,
stimulation of gene expression and protein synthesis, and ultimately a
long-lasting increase in the sensitivity of the postsynaptic neuron to glutamate
(step 7). This second-messenger system can also activate long-term
enhancement of presynaptic glutamate release via retrograde signals that have
not yet been identified (step 8). Each subsequent action potential arriving
along this presynaptic cell will cause a greater depolarization of the
postsynaptic membrane. Thus, repeatedly and intensely activating a particular
pattern of synaptic firing (as you might when studying for an exam) causes
chemical and structural changes that facilitate future activity along those same
pathways (as might occur when recalling what you learned).
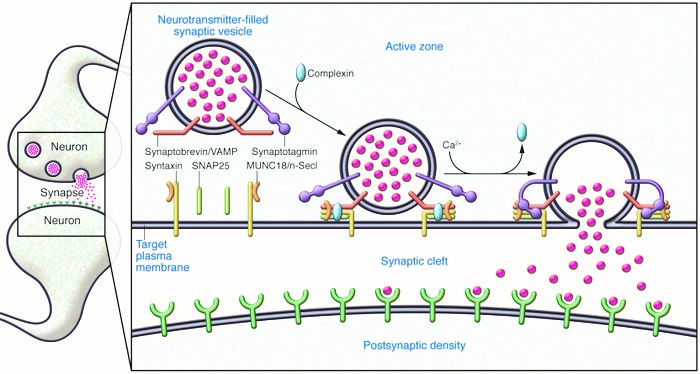
Prior to exocytosis, the synaptic vesicles are filled with neurotransmitter and
translocate to the active zone, where they dock at morphologically defined sites
on the target plasma membrane. The v-SNARE synaptobrevin/VAMP faces the target
plasma membrane, which contains the v-SNAREs SNAP25 and syntaxin, which
associates with MUNC18/n-Sec1. During the priming stage of vesicle fusion, the
SNARE proteins partially zipper together and complexin clamps the SNARE complex
in an activation-poised state to prevent membrane fusion. Action
potential–induced calcium influx triggers calcium, phospholipid, and SNARE
complex binding by synaptotagmin, which causes displacement of complexin and
opening of the fusion pore. Vesicle/target membrane fusion allows
neurotransmitter to enter the synaptic cleft and interact with the postsynaptic
density of the partner neuron.
(vSNARE- Vesicle SNARE; t-SNARE – Target SNARE; Q-SNARE – Gln SNARE; R-SNARE-Arg
SNARE; Munc18-Mammalian uncoordinated 18 protein)
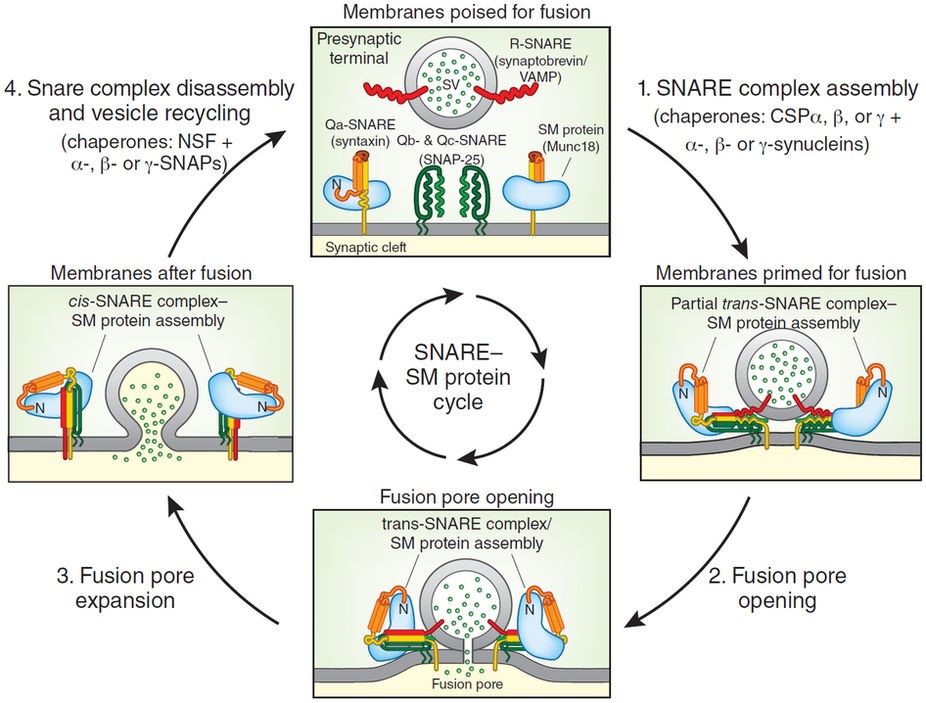
During step 1, synaptic vesicles are primed for fusion; this step involves
opening of the closed conformation of syntaxin, a switch of the Munc18-binding
mode of syntaxin from the closed to the open conformation and partial assembly
of trans-SNARE complexes. Step 1 is facilitated by recently discovered
chaperones (cysteine string proteins (CSPs) and synucleins) that enhance SNARE
complex assembly and whose dysfunction is related to neurodegeneration3.
During step 2, the fusion pore opens, with full trans-SNARE complex
assembly. During step 3, the fusion pore expands, converting trans-SNARE
into cis-SNARE complexes. In step 4, NSF and SNAPs mediate disassembly of
the SNARE complex, leading to vesicle recycling. The cycle shown here for
synaptic vesicle fusion is paradigmatic for most cytoplasmic fusion reactions,
although the details differ. In knockout experiments, deletion of the SM protein
Munc18-1 produces the most severe phenotype22,
possibly because loss of SNARE components of the fusion machinery is better
compensated for than loss of SM proteins. SNAREs are generally classified into
four types (R, Qa, Qb and Qc) that assemble into SNARE complexes in an
obligatory R-Qa-Qb-Qc combination.
NMDA receptors have also been implicated in mediating excitotoxicity.
This is a phenomenon in which the injury or death of some brain cells (due, for
example, to blocked or ruptured blood vessels) rapidly spreads to adjacent
regions. When glutamate-containing cells die and their membranes rupture, the
flood of glutamate excessively stimulates AMPA and NMDA receptors on nearby
neurons. The excessive stimulation of those neurons causes the accumulation of
toxic concentrations of intracellular Ca2+,
which in turn kills those neurons and causes them to rupture, and the
wave of damage progressively spreads. Recent experiments and clinical trials
suggest that administering NMDA receptor antagonists may help minimize the
spread of cell death following injuries to the brain.
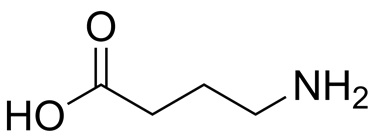
GABA
GABA
(gamma-aminobutyric acid) is the major inhibitory neurotransmitter in the
brain. Although it is not one of the 20 amino acids used to build proteins, it
is classified with the amino acid neurotransmitters because it is a modified
form of glutamate. With few exceptions, GABA neurons in the brain are small
interneurons that dampen activity within neural circuits. Postsynaptically, GABA
may bind to ionotropic or metabotropic receptors. The ionotropic receptor
increases Cl2 flux into the cell, resulting in hyperpolarization (an IPSP) of
the postsynaptic membrane. In addition to the GABA binding site, this receptor
has several additional binding sites for other compounds, including steroids,
barbiturates, and benzodiazepines. Benzodiazepine drugs such as alprazolam
(Xanax) and diazepam (Valium) reduce anxiety, guard
against seizures, and induce sleep by increasing Cl2 flux through the GABA
receptor. Synapses that use GABA are also among the many targets of the ethanol
(ethyl alcohol) found in alcoholic beverages. Ethanol stimulates GABA synapses
and simultaneously inhibits excitatory glutamate synapses, with the overall
effect being global depression of the electrical activity of the brain. Thus, as
a person’s blood alcohol content increases, there is a progressive reduction in
overall cognitive ability, along with sensory perception inhibition (hearing and
balance, in particular), loss of motor coordination, impaired judgment, memory
loss, and unconsciousness. Very high doses of ethanol are sometimes fatal, due
to suppression of brainstem centers responsible for regulating the circulatory
and respiratory systems. Dopaminergic and endogenous opioid signaling pathways
(discussed in the next section) are also affected by ethanol, which results in
short-term mood elevation or euphoria. The involvement of these pathways
underlies the development of long-term alcohol dependence in some people.
Glycine
Glycine
is the major neurotransmitter released from inhibitory interneurons in the
spinal cord and brainstem. It binds to ionotropic receptors on postsynaptic
cells that allow Cl2 to enter, thus preventing them from approaching
the threshold for firing action potentials. Normal function of glycinergic
neurons is essential for maintaining a balance of excitatory and inhibitory
activity in spinal cord integrating centers that regulate skeletal muscle
contraction. This becomes apparent in cases of poisoning with the neurotoxin
strychnine, an antagonist of glycine receptors sometimes used to kill
rodents. Victims experience hyperexcitability throughout the nervous system,
which leads to convulsions, spastic contraction of skeletal muscles, and
ultimately death due to impairment of the muscles of respiration.
Neuropeptides
The neuropeptides are composed of two or more amino acids linked together
by peptide bonds. About 100 neuropeptides have been identified, but their
physiological functions are not all known. It seems that evolution has favored
the same chemical messengers for use in widely differing circumstances, and many
of the neuropeptides have been previously identified in nonneural tissue where
they function as hormones or paracrine substances. They generally retain the
name they were given when first discovered in the nonneural tissue. The
neuropeptides are formed differently than other neurotransmitters, which are
synthesized in the axon terminals by very few enzyme-mediated steps. The
neuropeptides, in contrast, are derived from large precursor proteins, which in
themselves have little, if any, inherent biological activity. The synthesis of
these precursors, directed by mRNA, occurs on ribosomes, which exist only in the
cell body and large dendrites of the neuron, often
a
considerable distance from axon terminals or varicosities where the peptides are
released. In the cell body, the precursor protein is packaged into vesicles,
which are then moved by axonal transport into the terminals or varicosities,
where the protein is cleaved by specific peptidases. Many of the precursor
proteins contain multiple peptides, which may be different or be copies of one
peptide. Neurons that release one or more of the peptide neurotransmitters
are collectively called peptidergic. In many cases, neuropeptides are
cosecreted with another type of neurotransmitter and act as neuromodulators. The
amount of neuropeptide released from vesicles at synapses is significantly less
than the amount of nonpeptidergic neurotransmitters such as catecholamines. In
addition, neuropeptides can diffuse away from the synapse and affect other
neurons at some distance, in which case they are referred to as neuromodulators.
The actions of these neuromodulators are longer lasting (on the order of several
hundred milliseconds) than when neuropeptides or other molecules act as
neurotransmitters. After release, neuropeptides can interact with either
ionotropic or metabotropic receptors. They are eventually broken down by
peptidases located in neuronal membranes. Endogenous opioids—a group of
neuropeptides that includes beta-endorphin, the dynorphins, and
the enkephalins— have attracted much interest because their receptors are
the sites of action of opiate drugs such as morphine and
codeine. The opiate drugs are powerful analgesics (that
is, they relieve pain without loss of consciousness), and the endogenous opioids
undoubtedly have a function in regulating pain. There is also evidence that the
opioids function in regulating eating and drinking behavior, circulatory system
function, and mood and emotion.
Gases
Certain very short-lived gases also serve as neurotransmitters. Nitric oxide
is the best understood, but recent research indicates that carbon
monoxide and hydrogen sulfide are also emitted by neurons as signals.
Gases are not released by exocytosis of presynaptic vesicles, nor do they bind
to postsynaptic plasma membrane receptors. They are produced by enzymes in axon
terminals (in response to Ca21 entry) and simply diffuse from their sites of
origin in one cell into the intracellular fluid of other neurons or effector
cells, where they bind to and activate proteins. For example, nitric oxide
released from neurons activates guanylyl cyclase in recipient cells. This enzyme
increases the concentration of the second-messenger cyclic GMP, which in turn
can alter ion channel activity in the postsynaptic cell.
Nitric oxide functions in a bewildering array of neutrally mediated
events—learning, development, drug tolerance, penile and clitoral erection, and
sensory and motor modulation, to name a few. Paradoxically, it is also
implicated in neural damage that results, for example, from the stoppage of
blood flow to the brain or from a head injury. In later chapters, we will see
that nitric oxide is produced not only in the central and peripheral nervous
systems but also by a variety of nonneural cells; for example, it has important
paracrine functions in the circulatory and immune systems, among others.
Purines
Other nontraditional neurotransmitters include the purines, ATP and
adenosine, which act principally as neuromodulators. ATP is present in all
presynaptic vesicles and is coreleased with one or more other neurotransmitters
in response to Ca2+ influx into the terminal. Adenosine is derived
from ATP via enzyme activity occurring in the extracellular compartment. Both
presynaptic and postsynaptic receptors have been described for adenosine, and
the functions these substances have in the nervous system and other tissues are
active areas of research.
Neuroeffector Communication
Many neurons of the PNS end, however, not at synapses on other neurons but at
neuroeffector junctions on muscle, gland, and other cells. The neurotransmitters
released by these efferent neurons’ terminals or varicosities provide the link
by which electrical activity of the nervous system regulates effector cell
activity. The events that occur at neuroeffector junctions are similar to those
at synapses between neurons. The neurotransmitter is released from the efferent
neuron upon the arrival of an action potential at the neuron’s axon terminals or
varicosities. The neurotransmitter then diffuses to the surface of the effector
cell, where it binds to receptors on that cell’s plasma membrane. The receptors
may be directly under the axon terminal or varicosity, or they may be some
distance away so that the diffusion path the neurotransmitter follows is long.
The receptors on the effector cell may be either ionotropic or metabotropic.
Nerve impulse
The changes in Na+ and K+ diffusion and the resulting changes in the membrane
potential they produce constitute an event called the action potential,
or nerve impulse. The
electrochemical wave that travels along nerve fiber and stimulates muscles,
glands or other nerve cells is called as nerve impulse.
Spike Potential
The periodic rise of depolarization wave and rapid fall of repolarization
wave are known as spike potential.
All-or-None Law
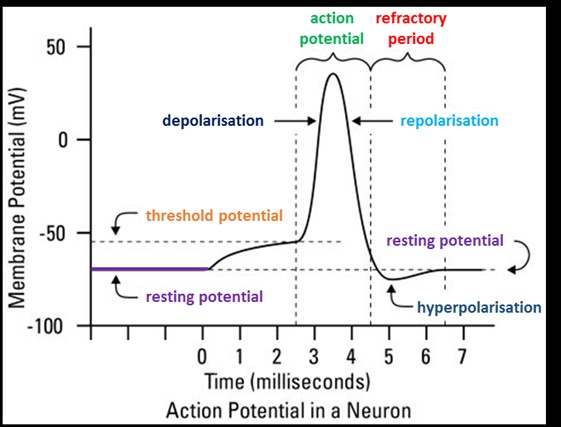
Once a region of axon membrane has been depolarized to a threshold value, the
positive feedback effect of depolarization on Na+ permeability and of Na+
permeability on depolarization causes the membrane potential to shoot toward
about +30 mV. It does not normally become more positive than +30 mV because the
Na+ channels quickly close and the K+ channels open. The length of time that the
Na+ and K+ channels stay open is independent of the strength of the
depolarization stimulus. The
amplitude (size) of action potentials is therefore all or none. When
depolarization is below a threshold value, the voltage-regulated gates are
closed; when depolarization reaches threshold, a maximum potential change (the
action potential) is produced. Because the change from −70 mV to +30 mV and back
to −70 mV lasts only about 3 msec, the image of an action potential on an
oscilloscope screen looks like a spike. Action potentials are therefore
sometimes called spike potentials. The channels are open only for a fixed
period of time because they are soon inactivated, a process different
from simply closing the gates. Inactivation occurs automatically and lasts until
the membrane has repolarized. Because of this automatic inactivation, all action
potentials have about the same duration. Likewise, since the concentration
gradient for Na+ is relatively constant, the amplitudes of the action potentials
are about equal in all axons at all times (from −70 mV to +30 mV, or about 100
mV in total amplitude).
Conduction of Nerve Impulses
When stimulating electrodes artificially depolarize one point of an axon
membrane to a threshold level, voltage-regulated channels open and an action
potential is produced at that small region of axon membrane containing those
channels. For about the first millisecond of the action potential, when the
membrane voltage changes from −70 mV to +30 mV, a current of Na+ enters the cell
by diffusion because of the opening of the Na+ gates. Each action potential thus
“injects” positive charges (sodium ions) into the axon. These positively charged
sodium ions are conducted, by the cable properties of the axon, to an adjacent
region that still has a membrane potential of −70 mV. Within the limits of the
cable properties of the axon (1 to 2 mm), this helps to depolarize the adjacent
region of axon membrane. When this adjacent region of membrane reaches a
threshold level of depolarization, it too produces the action potential as its
voltage-regulated gates open. The action potential produced at the first
location in the axon membrane (usually at the axon hillock) thus serves as the
depolarization stimulus for the next region of the axon membrane, which can then
produce the action potential. The action potential in this second region, in
turn, serves as a depolarization stimulus for the production of the action
potential in a third region, and so on. This explains how the action potential
is produced at all regions of the axon beyond the initial segment at the axon
hillock.
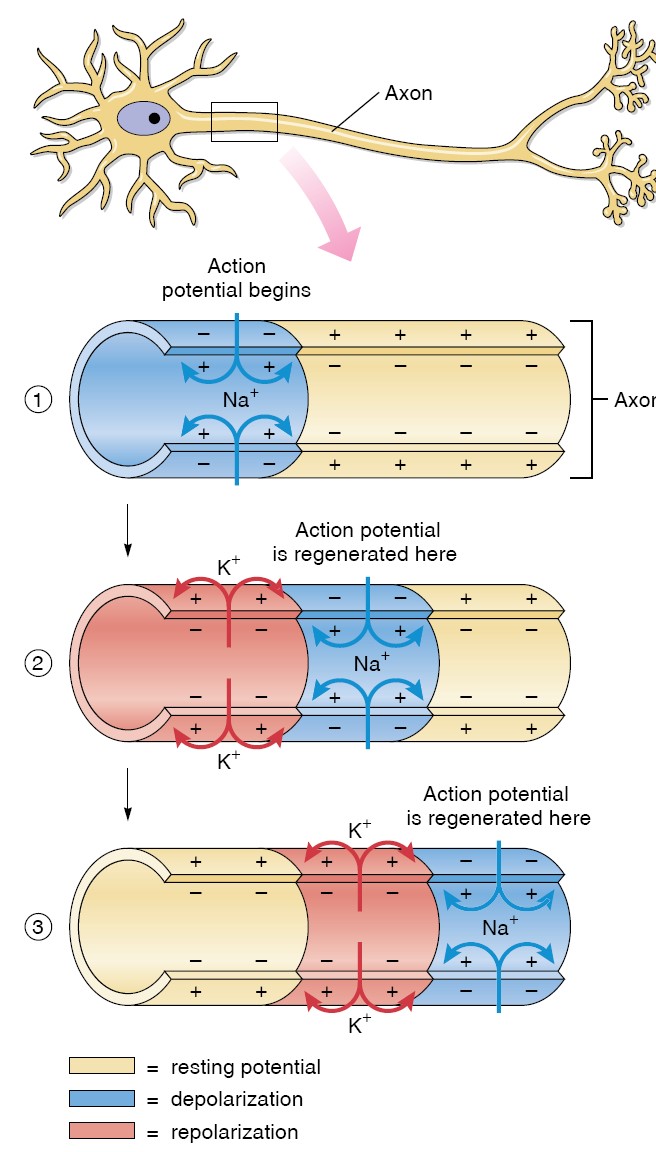
Conduction in an Unmyelinated Axon
In an unmyelinated axon, every patch of membrane that contains Na+ and K+
channels can produce an action potential. Action potentials are thus produced
along the entire length of the axon. The cablelike spread of depolarization
induced by the influx of Na + during one action potential helps to depolarize
the adjacent regions of membrane—a process that is also aided by movements of
ions on the outer surface of the axon membrane. This process would depolarize
the adjacent membranes on each side of the region to produce the action
potential, but the area that had previously produced one cannot produce another
at this time because it is still in its refractory period. It is important to
recognize that action potentials are not really “conducted,” although it is
convenient to use that word. Each action potential is a separate, complete event
that is repeated, or regenerated, along the axon’s length. This is
analogous to the “wave” performed by spectators in a stadium.
One person after another gets up (depolarization) and then sits down
(repolarization). It is thus the “wave” that travels (the repeated action
potential at different locations along the axon membrane), not the people.
The action potential produced at the end of the axon is thus a completely new
event that was produced in response to depolarization from the previous region
of the axon membrane. The action potential produced at the last region of the
axon has the same amplitude as the action potential produced at the first
region. Action potentials are thus said to be conducted without decrement
(without decreasing in amplitude). The spread of depolarization by the cable
properties of an axon is fast compared to the time it takes to produce an action
potential. Thus, the more action potentials along a given stretch of axon that
have to be produced, the slower the conduction. Because action potentials must
be produced at every fraction of a micrometer in an unmyelinated axon, the
conduction rate is relatively slow. This conduction rate is somewhat faster if
the unmyelinated axon is thicker, because thicker axons have less resistance to
the flow of charges (so conduction of charges by cable properties is faster).
The conduction rate is substantially faster if the axon is myelinated, because
fewer action potentials are produced along a given length of myelinated axon.
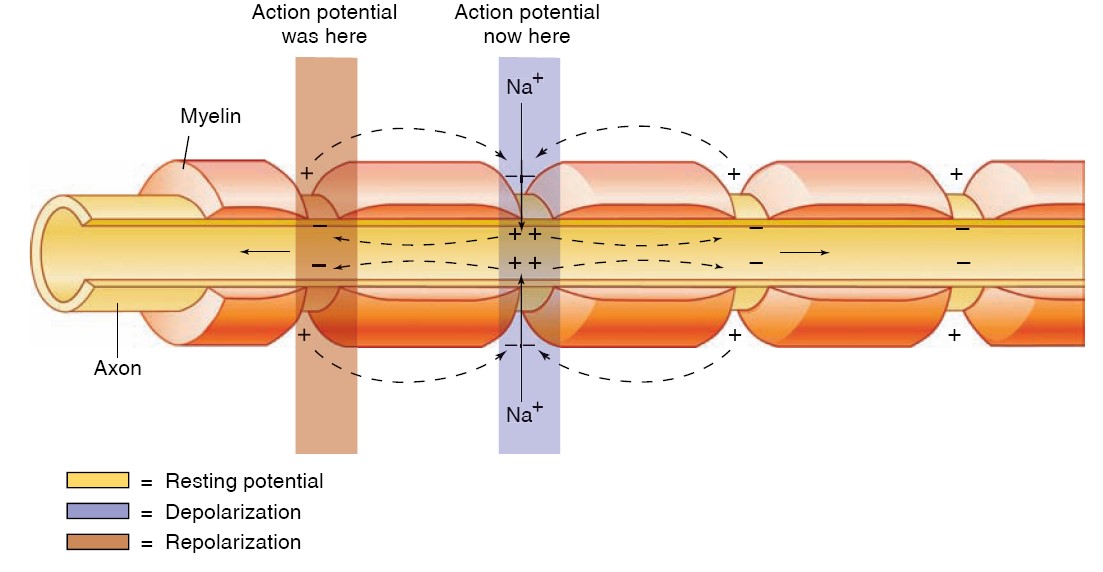
Conduction in a Myelinated Axon
The myelin sheath provides insulation for the axon, preventing movements of Na+
and K+ through the membrane. If the myelin sheath were continuous, therefore,
action potentials could not be produced. The myelin thus has interruptions-the
nodes of Ranvier, as previously described. Because the cable properties
of axons can conduct depolarizations over only a very short distance (1 to 2
mm), the nodes of Ranvier cannot be separated by more than this distance.
Studies have shown that Na+ channels are highly concentrated at the nodes
(estimated at 10,000 per square micrometer) and almost absent in the regions of
axon membrane between the nodes. Action potentials, therefore, occur only at the
nodes of Ranvier and seem to “leap” from node to node—a process called
saltatory conduction (from the Latin saltario = leap). The leaping
is, of course, just a metaphor; the action potential at one node depolarizes the
membrane at the next node to threshold, so that a new action potential is
produced at the next node of Ranvier.
Myelinated axons conduct the action potential faster than unmyelinated axons.
This is because myelinated axons have voltage-gated channels only at the nodes
of Ranvier, which are about 1 mm apart, whereas unmyelinated axons have these
channels along their entire length. Because myelinated axons have more cablelike
spread of depolarization (which is faster), and fewer sites at which the action
potential is produced (which is slower) than unmyelinated axons, the conduction
is faster in a myelinated axon. Conduction rates in the human nervous system
vary from 1.0 m/sec—in thin, unmyelinated fibers that mediate slow, visceral
responses-to faster than 100 m/sec (225 miles per hour)—in thick, myelinated
fibers involved in quick stretch reflexes in skeletal muscles.
In summary, the speed of action potential conduction is increased by (1)
increased diameter of the axon, because this reduces the resistance to the
spread of charges by cable properties; and (2) myelination, because the myelin
sheath results in saltatory conduction of action potentials. These methods of
affecting conduction speed are generally combined in the nervous system: the
thinnest axons tend to be unmyelinated and the thickest tend to be myelinated.
AUTONOMIC NERVOUS SYSTEM (ANS)
The autonomic nervous system (ANS) is the part of the nervous system that is
responsible for homeostasis. Except for skeletal muscle, which gets its
innervation from the somatomotor nervous system, innervation to all other organs
is supplied by the ANS. Nerve terminals are located in smooth muscle (eg, blood
vessels, the wall of the gastrointestinal tract, and urinary bladder), cardiac
muscle, and glands (eg, sweat glands and salivary glands). Although survival is
possible without an ANS, the ability to adapt to environmental stressors and
other challenges is severely compromised. The importance of understanding the
functions of the ANS is underscored by the fact that so many drugs used to treat
a vast array of diseases exert their actions on elements of the ANS. Also, many
neurologic diseases or disorders result directly from a loss of preganglionic
sympathetic neurons (eg, multiple system atrophy and Shy–Drager syndrome) and
other common diseases (eg, Parkinson disease and diabetes) are associated with
autonomic dysfunction.
The ANS has two major and anatomically distinct divisions: the sympathetic
and parasympathetic nervous systems. As will be described, some
target organs are innervated by both divisions and others are controlled by only
one. In addition, the ANS includes the enteric nervous system within the
gastrointestinal tract. The classic definition of the ANS is the preganglionic
and postganglionic neurons within the sympathetic and parasympathetic divisions.
This would be equivalent to defining the somatomotor nervous system as the
cranial and spinal motor neurons. A modern definition of the ANS takes into
account the descending pathways from several forebrain and brainstem regions as
well as visceral afferent pathways that set the level of activity in sympathetic
and parasympathetic nerves. This is analogous to including the many descending
and ascending pathways that influence the activity of somatic motor neurons as
elements of the somatomotor nervous system. The sympathetic divisions
responsible for showing, expressing and feeling like fight-flight-fright
response. The parasympathetic
divisions responsible for rest state and digestion. It is contrary to
sympathetic nervous system function.
SYMPATHETIC DIVISION
In contrast to α-motor neurons, which are located at all spinal segments,
sympathetic preganglionic neurons are located in the IML of only the first
thoracic to the third or fourth lumbar segments. This is why the sympathetic
nervous system is sometimes called the thoracolumbar division of the ANS. The
axons of the sympathetic preganglionic neurons leave the spinal cord at the
level at which their cell bodies are located and exit via the ventral root along
with axons of α- and γ-motor neurons. They then separate from the ventral root
via the white rami communicans and project to the adjacent sympathetic
paravertebral ganglion, where some of them end on the cell bodies of the
postganglionic neurons. Paravertebral ganglia are located adjacent to each
thoracic and upper lumbar spinal segment; in addition, there are a few ganglia
adjacent to the cervical and sacral spinal segments. The ganglia are connected
to each other via the axons of preganglionic neurons that travel rostrally or
caudally to terminate on postganglionic neurons located at some distance.
Together these ganglia and axons form the sympathetic chain bilaterally.
Some preganglionic neurons pass through the paravertebral ganglion chain and
end on postganglionic neurons located in prevertebral (or collateral)
ganglia close to the viscera, including the celiac, superior mesenteric,
and inferior mesenteric ganglia. There are also preganglionic neurons whose
axons terminate directly on the effector organ, the adrenal gland.
The axons of some of the postganglionic neurons leave the chain ganglia
and reenter the spinal nerves via the gray rami communicans and are
distributed to autonomic effectors in the areas supplied by these spinal
nerves. These postganglionic sympathetic nerves terminate mainly on smooth
muscle (eg, blood vessels and hair follicles) and on sweat glands in the limbs.
Other postganglionic fibers leave the chain ganglia to enter the thoracic cavity
to terminate in visceral organs. Postganglionic fibers from prevertebral
ganglia also terminate in visceral targets.
PARASYMPATHETIC DIVISION
The parasympathetic nervous system is sometimes called the craniosacral
division of the ANS because of the location of its preganglionic neurons;
preganglionic neurons are located in several cranial nerve nuclei (III, VII, IX,
and X) and in the IML of the sacral spinal cord. The cell bodies in the
Edinger–Westphal nucleus of the oculomotor nerve project to the ciliary
ganglia to innervate the sphincter (constrictor) muscle of the iris and the
ciliary muscle. Neurons in the superior salivatory nucleus of the facial
nerve project to the sphenopalatine ganglia to innervate the lacrimal
glands and nasal and palatine mucous membranes and to the submandibular
ganglia to innervate the submandibular (also called submaxillary) and
sublingual glands. The cell bodies in the inferior salivatory nucleus of
the glossopharyngeal nerve project to the otic ganglion to innervate
the parotid salivary gland. Vagal preganglionic fibers synapse on ganglia cells
clustered within the walls of visceral organs; thus these parasympathetic
postganglionic fibers are very short. Neurons in the nucleus ambiguus
innervate the sinoatrial (SA) and atrioventricular (AV) nodes in the heart; and
neurons in the dorsal motor vagal nucleus innervate the esophagus,
trachea, lungs, and gastrointestinal tract. The parasympathetic sacral outflow
(pelvic nerve) supplies the pelvic viscera via branches of the second to
fourth sacral spinal nerves.
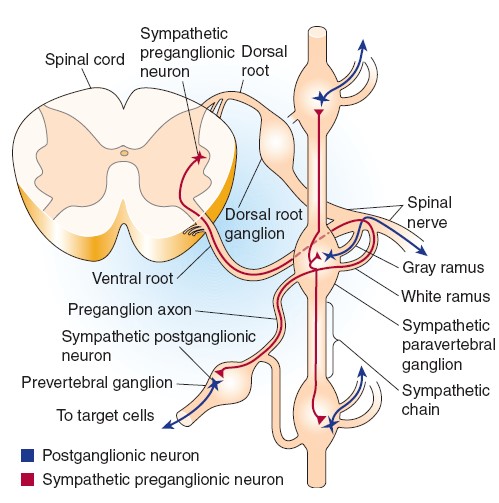
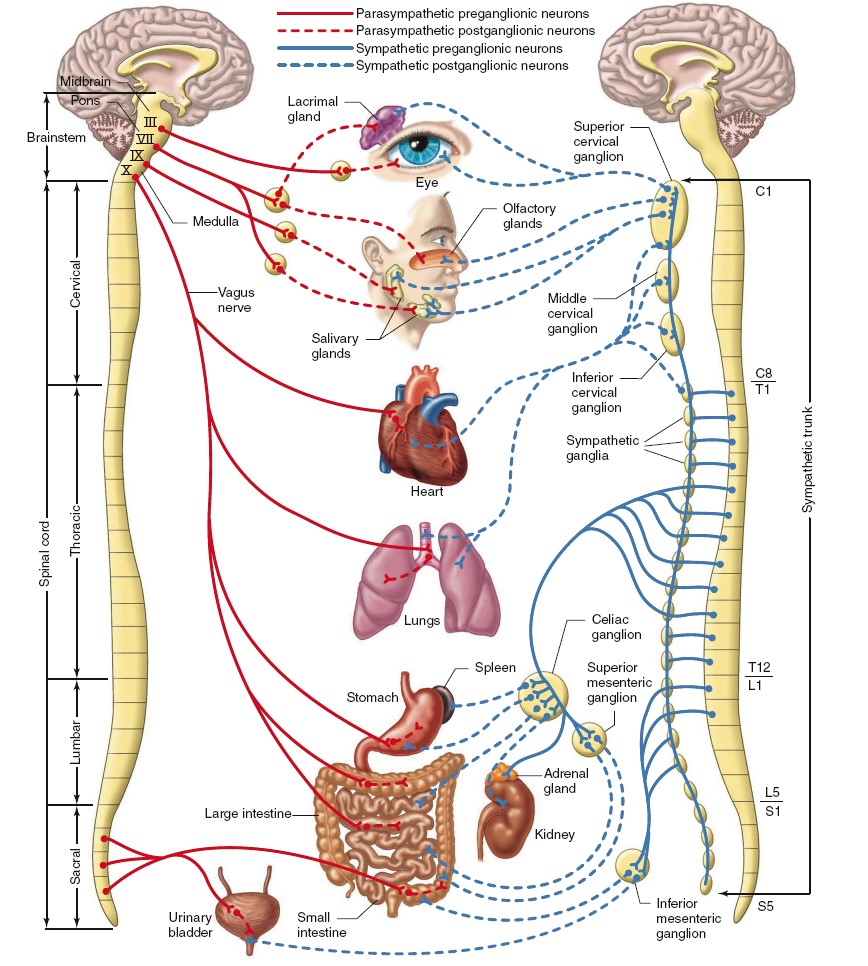
The parasympathetic (at left) and sympathetic (at right) divisions of the
autonomic nervous system. Although single nerves are shown exiting the brainstem
and spinal cord, all represent paired (left and right) nerves. Only one
sympathetic trunk is indicated, although there are two, one on each side of the
spinal cord. The celiac, superior mesenteric, and inferior mesenteric ganglia
are collateral ganglia. Not shown are the fibers passing to the liver, blood
vessels, genitalia, and skin glands.
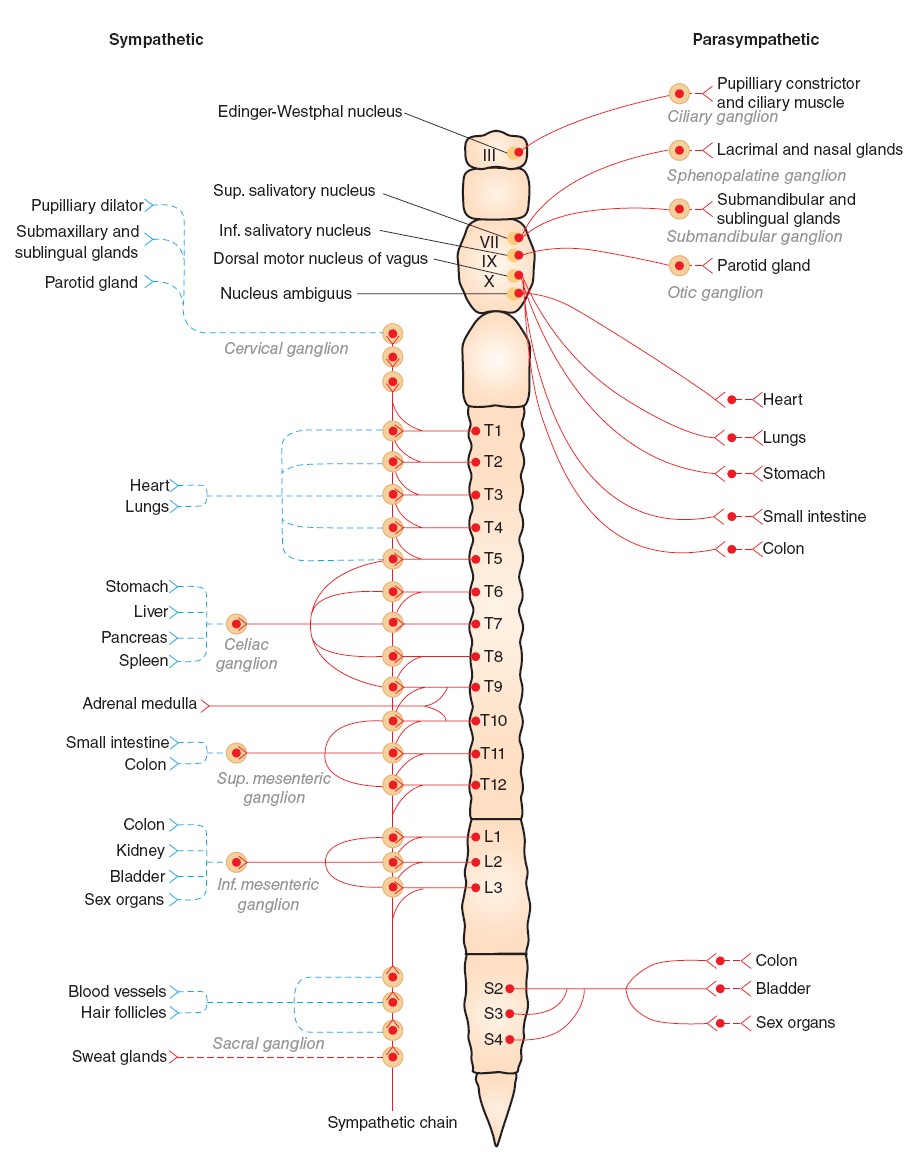
GENERAL
PROPERTIES OF REFLEXES
The basic unit of integrated reflex activity is the reflex arc. This arc
consists of a sense organ, an afferent neuron, one or more synapses within a
central integrating station, an efferent neuron, and an effector. The afferent
neurons enter via the dorsal roots or cranial nerves and have their cell bodies
in the dorsal root ganglia or in the homologous ganglia of the cranial nerves.
The efferent fibers leave via the ventral roots or corresponding motor cranial
nerves. Activity in the reflex arc
starts in a sensory receptor with a receptor potential whose magnitude is
proportional to the strength of the stimulus. This generates all-or-none action
potentials in the afferent nerve, the number of action potentials being
proportional to the size of the receptor potential. In the central nervous
system (CNS), the responses are again graded in terms of excitatory postsynaptic
potentials (EPSPs) and inhibitory postsynaptic potentials (IPSPs) at the
synaptic junctions. All-or-none responses (action potentials) are generated in
the efferent nerve. When these reach the effector, they again set up a graded
response. When the effector is smooth muscle, responses summate to produce
action potentials in the smooth muscle. In contrast, when the effector is
skeletal muscle, the graded response is adequate to produce action potentials
that bring about muscle contraction. The connection between the afferent and
efferent neurons is in the CNS, and activity in the reflex arc is modified by
the multiple inputs converging on the efferent neurons or at any synaptic
station within the reflex arc. The
stimulus that triggers a reflex is generally very precise. This stimulus is
called the adequate stimulus for the particular reflex. A dramatic
example is the scratch reflex in the dog. This spinal reflex is adequately
stimulated by multiple linear touch stimuli such as those produced by an insect
crawling across the skin. The response is vigorous scratching of the area
stimulated. If the multiple touch stimuli are widely separated or not in a line,
the adequate stimulus is not produced and no scratching occurs. Fleas crawl, but
they also jump from place to place. This jumping separates the touch stimuli so
that an adequate stimulus for the scratch reflex is not produced.
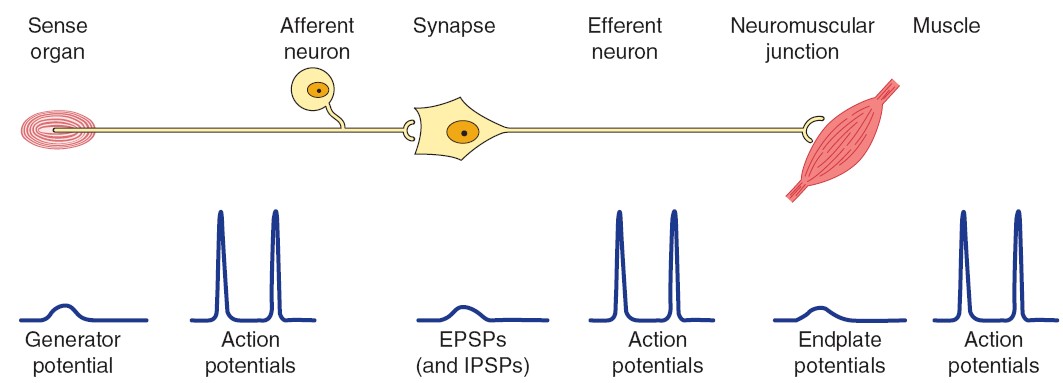
MONOSYNAPTIC REFLEXES: THE STRETCH REFLEX
The simplest reflex arc is one with a single synapse between the afferent and
efferent neurons, and reflexes occurring in them are called monosynaptic
reflexes. Reflex arcs in which interneurons are interposed between the
afferent and efferent neurons are called polysynaptic reflexes. There can
be anywhere from two to hundreds of synapses in a polysynaptic reflex arc.
When a skeletal muscle with an intact nerve supply is stretched, it
contracts. This response is called the stretch reflex or myotatic
reflex. The stimulus that initiates this reflex is stretch of the muscle,
and the response is contraction of the muscle being stretched. The sense organ
is a small encapsulated spindlelike or fusiform-shaped structure called the
muscle spindle, located within the fleshy part of the muscle. The impulses
originating from the spindle are transmitted to the CNS by fast sensory fibers
that pass directly to the motor neurons that supply the same muscle. The
neurotransmitter at the central synapse is glutamate. The stretch reflex is the
best known and studied monosynaptic reflex and is typified by the knee jerk
reflex.
MUSCLE SYSTEM
Muscle cells, like neurons, can be excited chemically, electrically, and
mechanically to produce an action potential that is transmitted along their cell
membranes. Unlike neurons, they respond to stimuli by activating a contractile
mechanism. The contractile protein myosin and the cytoskeletal protein actin are
abundant in muscle, where they are the primary structural components that bring
about contraction. Muscle is
generally divided into three types: skeletal, cardiac, and smooth,
although smooth muscle is not a homogeneous single category. Skeletal muscle
makes up the great mass of the somatic musculature. It has well-developed
cross-striations, does not normally contract in the absence of nervous
stimulation, lacks anatomic and functional connections between individual muscle
fibers, and is generally under voluntary control. Cardiac muscle also has
cross-striations, but it is functionally syncytial and, although it can be
modulated via the autonomic nervous system, it can contract rhythmically in the
absence of external innervation owing to the presence in the myocardium of
pacemaker cells that discharge spontaneously. Smooth muscle lacks
cross-striations and can be further subdivided into two broad types: unitary (or
visceral) smooth muscle and multiunit smooth muscle. The type found in most
hollow viscera is functionally syncytial and contains pacemakers that discharge
irregularly. The multiunit type found in the eye and in some other locations is
not spontaneously active and resembles skeletal muscle in graded contractile
ability.
SKELETAL
MUSCLE MORPHOLOGY
Skeletal muscle is made up of individual muscle fibers that are the “building
blocks” of the muscular system in the same sense that the neurons are the
building blocks of the nervous system. Most skeletal muscles begin and end in
tendons, and the muscle fibers are arranged in parallel between the tendinous
ends, so that the force of contraction of the units is additive. Each muscle
fiber is a single cell that is multinucleated, long, cylindrical, and surrounded
by a cell membrane, the sarcolemma. There are no syncytial bridges
between cells. The muscle fibers are made up of myofibrils, which are divisible
into individual filaments. These myofilaments contain several proteins that
together make up the contractile machinery of the skeletal muscle.
The contractile mechanism in skeletal muscle largely depends on the proteins
myosin-II, actin, tropomyosin, and troponin. Troponin is made up of
three subunits: troponin I, troponin T, and troponin C. Other
important proteins in muscle are involved in maintaining the proteins that
participate in contraction in appropriate structural relation to one another and
to the extracellular matrix.
microscope. The parts of the cross-striations are frequently identified by
letters. The light I band is divided by the dark Z line, and the dark A band has
the lighter H band in its center. A transverse M line is seen in the middle of
the H band, and this line plus the narrow light areas on either side of it are
sometimes called the pseudo-H zone. The area between two adjacent Z lines is
called a sarcomere. The orderly arrangement of actin, myosin, and related
proteins that produces this pattern. The thick filaments, which are about twice
the diameter of the thin filaments, are made up of myosin; the thin filaments
are made up of actin, tropomyosin, and troponin. The thick filaments are lined
up to form the A bands, whereas the array of thin filaments extends out of the A
band and into the less dense staining I bands. The lighter H bands in the center
of the A bands are the regions where, when the muscle is relaxed, the thin
filaments do not overlap the thick filaments. The Z lines allow for anchoring of
the thin filaments. If a transverse section through the A band is examined under
the electron microscope, each thick filament is seen to be surrounded by six
thin filaments in a regular hexagonal pattern.
The form of myosin found in muscle is myosin-II, with two globular heads and a
long tail. The heads of the myosin molecules form cross-bridges with actin.
Myosin contains heavy chains and light chains, and its heads are made up of the
light chains and the amino terminal portions of the heavy chains. These heads
contain an actin-binding site and a catalytic site that hydrolyzes adenosine
triphosphate (ATP). The myosin molecules are arranged symmetrically on either
side of the center of the sarcomere, and it is this arrangement that creates
the light areas in the pseudo-H zone. The M line is the site of the reversal of
polarity of the myosin molecules in each of the thick filaments. At these
points, there are slender cross-connections that hold the thick filaments in
proper array. Each thick filament contains several hundred myosin molecules.
The thin filaments are polymers made up of two chains of actin that form
a long double helix. Tropomyosin molecules are long filaments located in the
groove between the two chains in the actin. Each thin filament contains 300–400
actin molecules and 40–60 tropomyosin molecules. Troponin molecules are small
globular units located at intervals along the tropomyosin molecules. Each of the
three troponin subunits has a unique function: Troponin T binds the troponin
components to tropomyosin, troponin I inhibits the interaction of myosin with
actin, and troponin C contains the binding sites for the Ca2+ that helps
initiate contraction.
Some additional structural proteins that are important in skeletal muscle
function include actinin, titin, and desmin. Actinin binds actin
to the Z lines. Titin, the largest known protein (with a molecular mass near
3,000,000 Da), connects the Z lines to the M lines and provides scaffolding for
the sarcomere. It contains two kinds of folded domains that provide muscle with
its elasticity. At first when the muscle is stretched there is relatively little
resistance as the domains unfold, but with further stretch there is a rapid
increase in resistance that protects the structure of the sarcomere. Desmin adds
structure to the Z lines in part by binding the Z lines to the plasma membrane.
It should be noted that although these proteins are important in muscle
structure/function, by no means do they represent an exhaustive list.
SARCOTUBULAR SYSTEM
The muscle fibrils are surrounded by structures made up of membranes that appear
in electron micrographs as vesicles and tubules. These structures form the
sarcotubular system, which is made up of a T system and a
sarcoplasmic reticulum. The T system of transverse tubules, which is
continuous with the sarcolemma of the muscle fiber, forms a grid perforated by
the individual muscle fibrils. The space between the two layers of the T system
is an extension of the extracellular space. The sarcoplasmic reticulum, which
forms an irregular curtain around each of the fibrils, has enlarged terminal
cisterns in close contact with the T system at the junctions between the A
and I bands. At these points of contact, the arrangement of the central T system
with a cistern of the sarcoplasmic reticulum on either side has led to the use
of the term triads to describe the system. The T system, which is
continuous with the sarcolemma, provides a path for the rapid transmission of
the action potential from the cell membrane to all the fibrils in the muscle.
The sarcoplasmic reticulum is an important store of Ca2+ and also participates
in muscle metabolism.
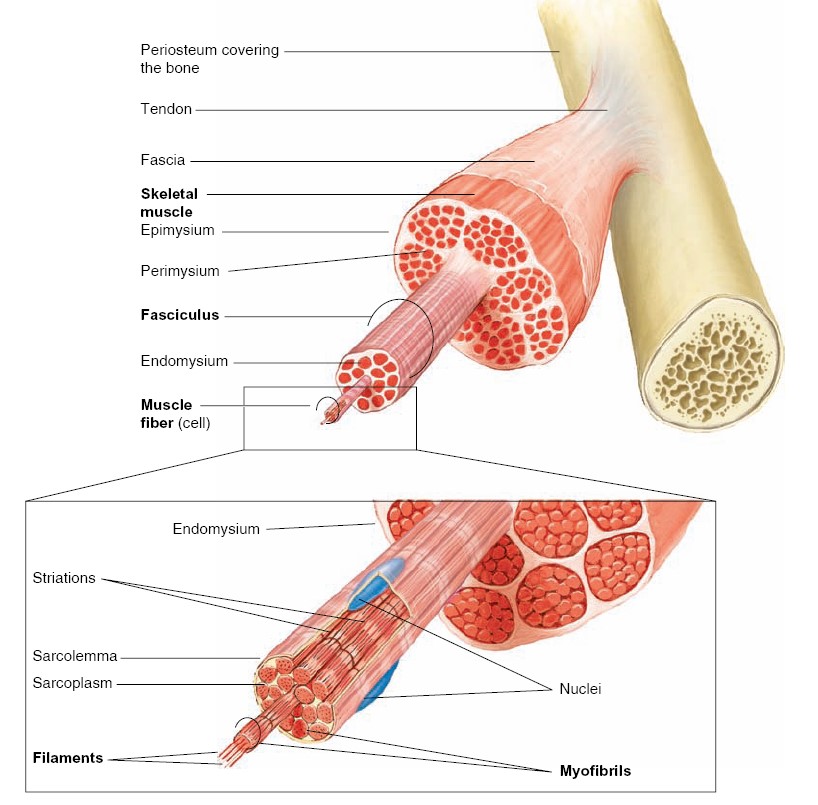
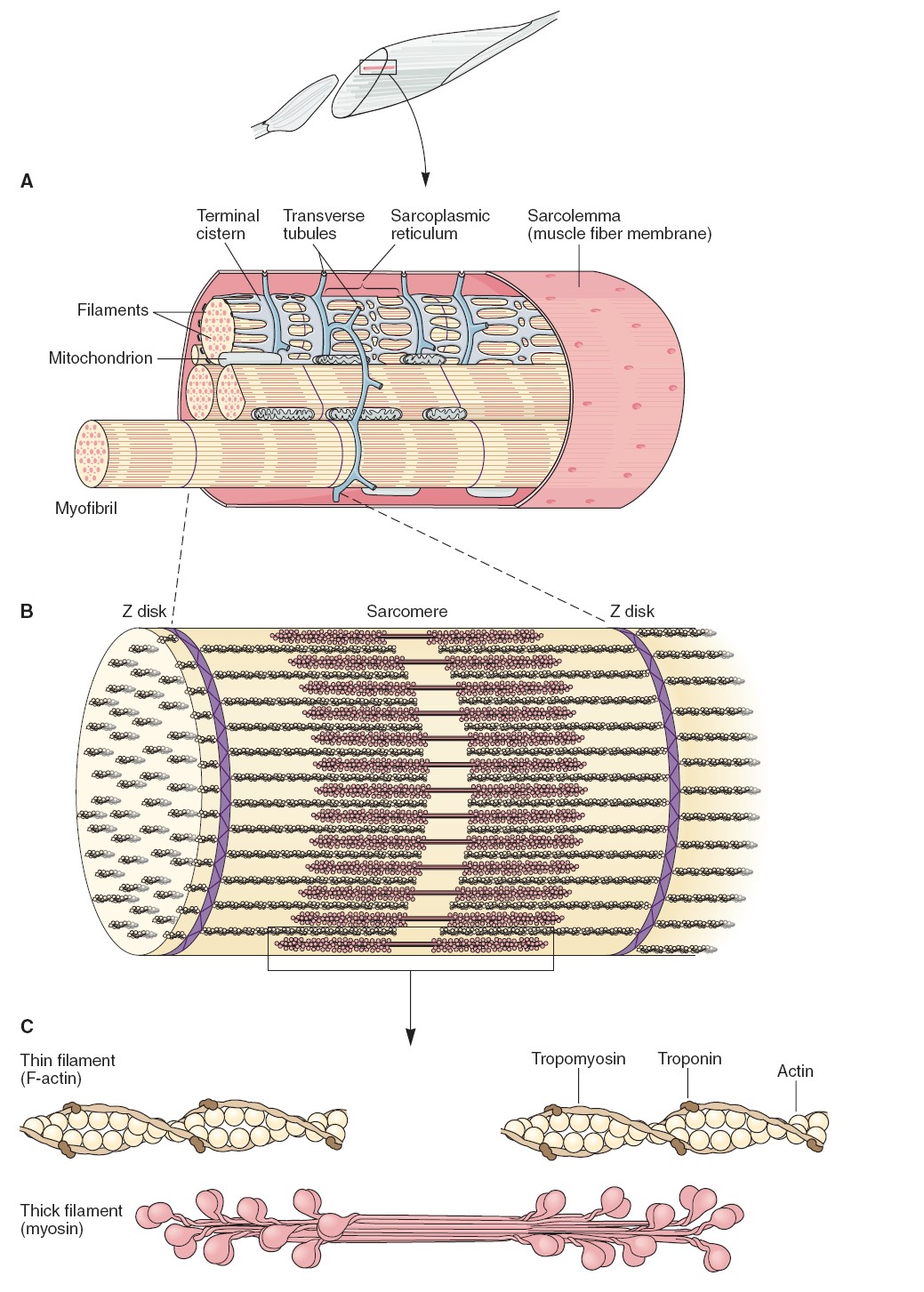
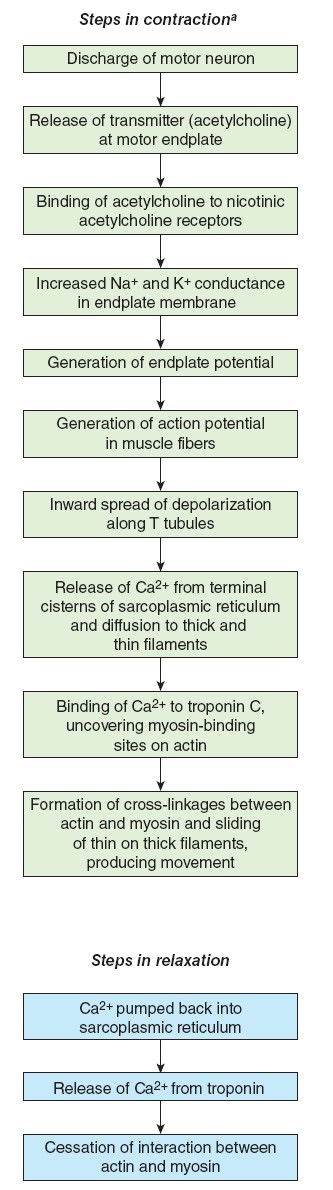
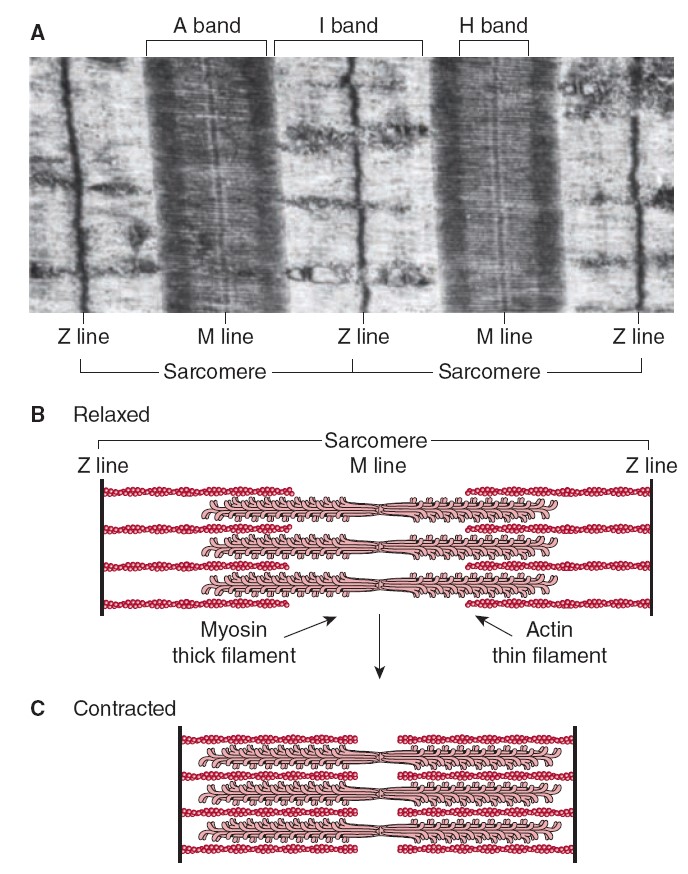
DYSTROPHIN–GLYCOPROTEIN COMPLEX
The large dystrophin protein (molecular mass 427,000 Da) forms a rod that
connects the thin actin filaments to the transmembrane protein
β-dystroglycan in the sarcolemma by smaller proteins in the cytoplasm,
syntrophins. β-dystroglycan is connected to merosin (merosin refers
to laminins that contain the α2 subunit in their trimeric makeup) in the
extracellular matrix by α-dystroglycan. The dystroglycans are in turn
associated with a complex of four transmembrane glycoproteins: α-, β-, γ-, and δ-sarcoglycan.
This dystrophin–glycoprotein complex adds strength to the muscle by
providing a scaffolding for the fibrils and connecting them to the
extracellular environment. Disruption of these important structural features can
result in several different muscular dystrophies.
MYOFIBRIL
When muscle cells are viewed in the electron microscope, which can produce
images at several thousand times the magnification possible in an ordinary light
microscope, each cell is seen to be composed of many subunits known as
myofibrils (fibrils = little fibers). These myofibrils are
approximately 1 micrometer (1 μ m) in diameter and extend in parallel rows from
one end of the muscle fiber to the other. The myofibrils are so densely packed
that other organelles, such as mitochondria and intracellular membranes, are
restricted to the narrow cytoplasmic spaces that remain between adjacent
myofibrils. When a muscle fiber is seen with an electron microscope, its
striations do not extend all the way across its width. Rather, the dark A
bands and light I bands that produce the striations are seen within
each myofibril. Because the dark and light bands of different myofibrils are
stacked in register (aligned vertically) from one side of the muscle fiber to
the other, and the individual myofibrils are not visible with an ordinary light
microscope, the entire muscle fiber seems to be striated under a light
microscope.
Each myofibril contains even smaller structures called myofilaments. When
a myofibril is observed at high magnification in longitudinal section (side
view), the A bands are seen to contain thick filaments. These are about
110 angstroms thick (110 Å, where 1 Å = 10 − 10 m) and are stacked in register.
It is these thick filaments that give the A band its dark appearance. The
lighter I band, by contrast, contains thin filaments (from 50 to 60 Å
thick). The thick filaments are primarily composed of the protein myosin,
and the thin filaments are primarily composed of the protein actin. The I
bands within a myofibril are the lighter areas that extend from the edge of one
stack of thick filaments to the edge of the next stack of thick filaments. They
are light in appearance because they contain only thin filaments. The thin
filaments, however, do not end at the edges of the I bands. Instead, each thin
filament extends partway into the A bands on each side (between the stack of
thick filaments on each side of an I band). Because thick and thin filaments
overlap at the edges of each A band, the edges of the A band are darker in
appearance than the central region. These central lighter regions of the A bands
are called the H bands (for helle, a German word meaning
“bright”). The central H bands thus contain only thick filaments that are not
overlapped by thin filaments. In the center of each I band is a thin dark Z
line. The arrangement of thick and thin filaments between a pair of Z lines
forms a repeating pattern that serves as the basic subunit of striated muscle
contraction. These subunits, from Z to Z, are known as sarcomeres. A
longitudinal section of a myofibril thus presents a side view of successive
sarcomeres. This side view is, in a sense, misleading; there are numerous
sarcomeres within each myofibril that are out of the plane of the section (and
out of the picture). A better appreciation of the three-dimensional structure of
a myofibril can be obtained by viewing the myofibril in cross section. In this
view, it can be seen that the Z lines are actually Z discs, and that the
thin filaments that penetrate these Z discs surround the thick filaments in a
hexagonal arrangement. If we
concentrate on a single row of dark thick filaments in this cross section, the
alternating pattern of thick and thin filaments seen in longitudinal section
becomes apparent. The M lines are produced by protein filaments located
at the center of the thick filaments (and thus the A band) in a sarcomere. These
serve to anchor the thick filaments, helping them to stay together during a
contraction. Also shown are
filaments of titin, a type of elastic protein that runs through the thick
filaments from the M lines to the Z discs. Because of its elastic properties,
titin is believed to contribute to the elastic recoil of muscles that helps them
to return to their resting length during muscle relaxation.
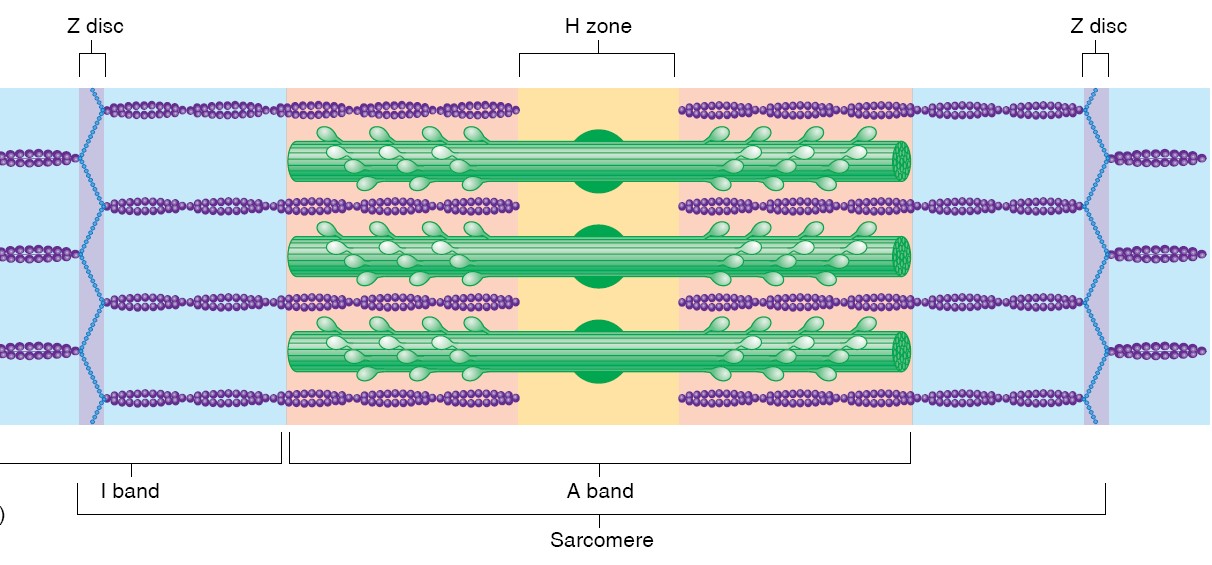
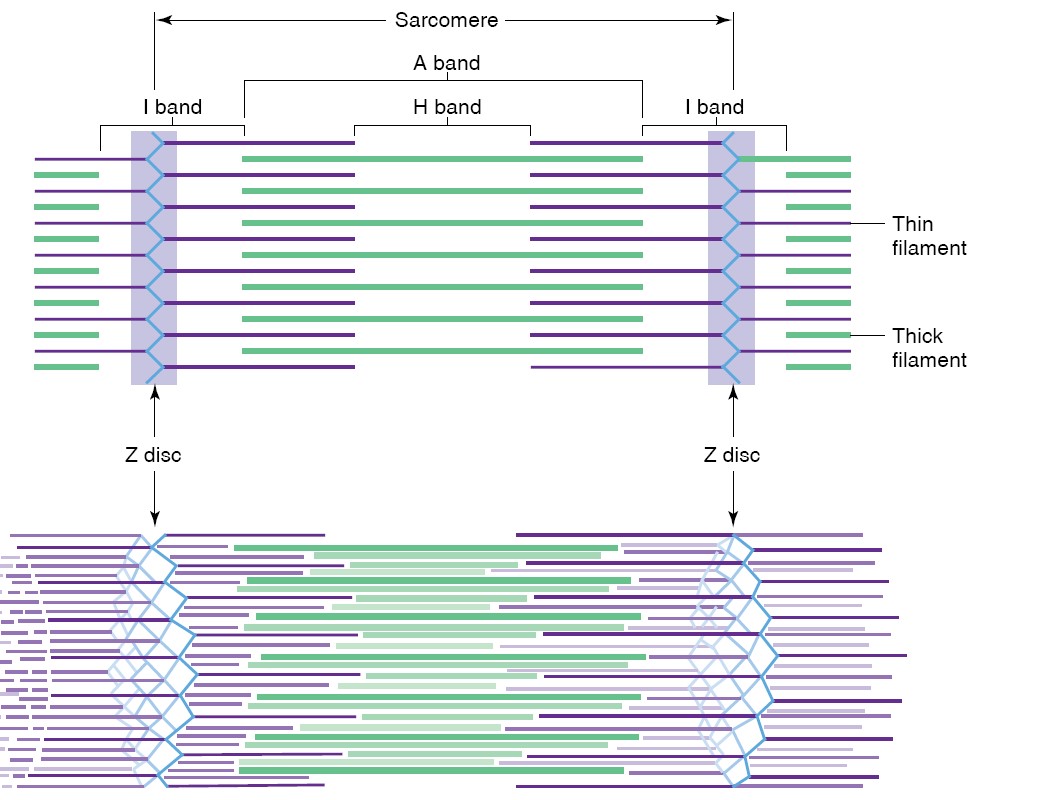
Sliding theory events:
1. A myofiber, together with all its myofibrils, shortens by movement of the
insertion toward the origin of the muscle.
2. Shortening of the myofibrils is caused by shortening of the sarcomeres—the
distance between Z lines (or discs) is reduced.
3. Shortening of the sarcomeres is accomplished by sliding of the
myofilaments—the length of each filament remains the same during contraction.
4. Sliding of the filaments is produced by asynchronous power strokes of myosin
cross bridges, which pull the thin filaments (actin) over the thick filaments
(myosin).
5. The A bands remain the same length during contraction, but are pulled toward
the origin of the muscle.
6. Adjacent A bands are pulled closer together as the I bands between them
shorten.
7. The H bands shorten during contraction as the thin filaments on the sides of
the sarcomeres are pulled toward the middle.
SLIDING FILAMENT THEORY OF CONTRACTION
When a muscle contracts it decreases in length as a result of the shortening of
its individual fibers. Shortening of the muscle fibers, in turn, is produced by
shortening of their myofibrils, which occurs as a result of the shortening of
the distance from Z disc to Z disc. As the sarcomeres shorten in length,
however,
the A bands do not shorten but instead move closer together. The I
bands—which represent the distance between A bands of successive
sarcomeres—decrease in length. The thin filaments composing the I band, however,
do not shorten. Close examination reveals that the thick and thin filaments
remain the same length during muscle contraction. Shortening of the sarcomeres
is produced not by shortening of the filaments, but rather by the sliding
of thin filaments over and between the thick filaments. In the process of
contraction, the thin filaments on either side of each A band slide deeper and
deeper toward the center, producing increasing amounts of overlap with the thick
filaments. The I bands (containing only thin filaments) and H bands (containing
only thick filaments) thus get shorter during contraction.
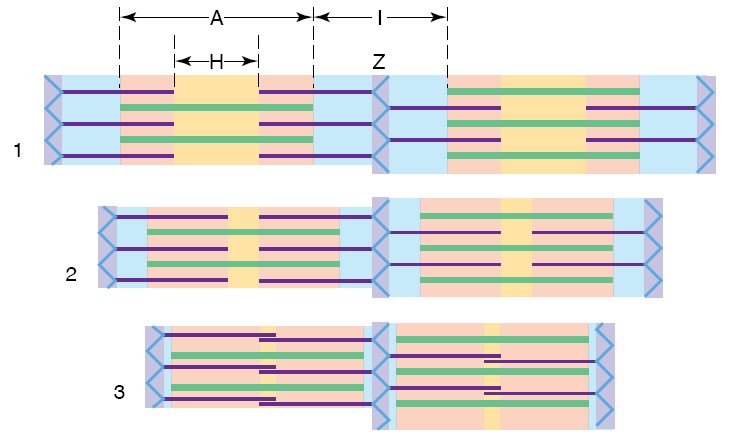
Cross Bridges
Sliding of the filaments is produced by the action of numerous cross bridges
that extend out from the myosin toward the actin. These cross bridges are
part of the myosin proteins that extend from the axis of the thick filaments to
form “arms” that terminate in globular “heads”. A myosin protein has two
globular heads that serve as cross bridges. The orientation of the myosin heads
on one side of a sarcomere is opposite to that of the other side, so that, when
the myosin heads form cross bridges by attaching to actin on each side of the
sarcomere, they can pull the actin from each side toward the center. Isolated
muscles are easily stretched (although this is opposed in the body by the
stretch reflex, described in a later section), demonstrating that the myosin
heads are not attached to actin when the muscle is at rest. Each globular myosin
head of a cross bridge contains an ATP-binding site closely associated
with an actin-binding site. The globular heads function as myosin
ATPase enzymes, splitting ATP into ADP and Pi. This reaction must occur
before the myosin heads can bind to actin. When ATP is hydrolyzed to ADP and
Pi, the phosphate binds to the myosin head, phosphorylating it and causing it to
change its conformation so that it becomes “cocked” (by analogy to the hammer of
a gun). The position of the myosin head has changed and it now has the potential
energy required for contraction. Perhaps a more apt analogy is with a bow and
arrow: The energized myosin head is like a pulled bowstring; it is now in
position to bind to actin, right) so that its stored energy can be
released in the next step. Once the myosin head binds to actin, forming a cross
bridge, the bound Pi is released (the myosin head becomes dephosphorylated).
This results in a conformational change in the myosin, causing the cross bridge
to produce a power stroke. This is the force that pulls the thin
filaments toward the center of the A band. After the power stroke, with the
myosin head now in its flexed position, the bound ADP is released as a new ATP
molecule binds to the myosin head. This release of ADP and binding to a new ATP
is required for the myosin head to break its bond with actin after the power
stroke is completed. The myosin head will then split ATP to ADP and Pi, and—if
nothing prevents the binding of the myosin head to the actin—a new cross-bridge
cycle will occur.
Note that the splitting of ATP is required before a cross bridge can
attach to actin and undergo a power stroke, and that the attachment of a new
ATP is needed for the cross bridge to release from actin at the end of a
power stroke. A single cross-bridge
power stroke pulls the actin filament a distance of 6 nanometers (6 nm), and all
of the cross bridges acting together in a single cycle will shorten the muscle
by less than 1% of its resting length. Muscles can shorten up to 60% of their
resting lengths, so the contraction cycles must be repeated many times. For this
to occur the cross bridges must detach from the actin at the end of a power
stroke, reassume their resting orientation, and then reattach to the actin and
repeat the cycle. During normal contraction, however, only a portion of the
cross bridges are attached at any given time. The power strokes are thus not in
synchrony, as the strokes of a competitive rowing team would be. Rather, they
are like the actions of a team engaged in tug-of-war, where the pulling action
of the members is asynchronous. Some cross bridges are engaged in power strokes
at all times during the contraction. The force produced by each power stroke is
constant, but when the muscle’s load is greater, the number of cross bridges
engaged in power strokes is increased to generate more force.
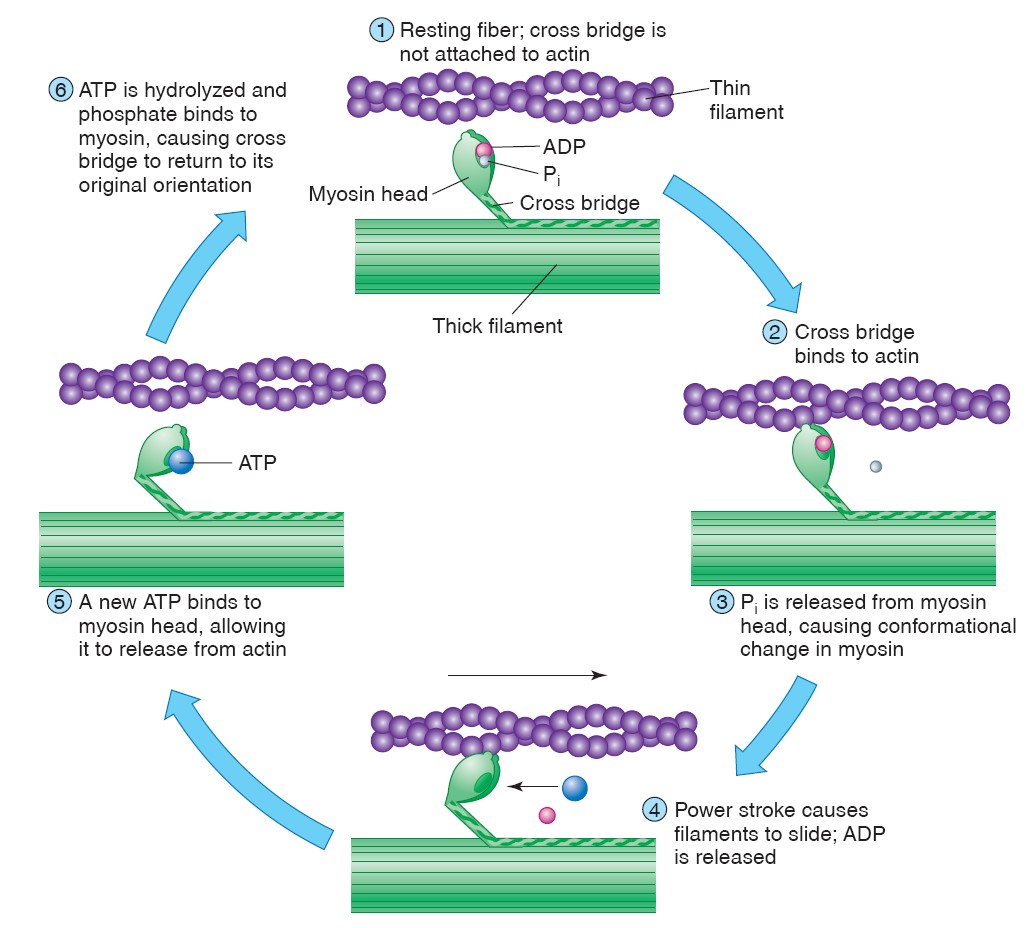
Regulation of Contraction
When the cross bridges attach to actin, they undergo power strokes and cause
muscle contraction. In order for a muscle to relax, therefore, the attachment of
myosin cross bridges to actin must be prevented. The regulation of cross-bridge
attachment to actin is a function of two proteins that are associated with actin
in the thin filaments. The actin filament—or F-actin —is a polymer formed
of 300 to 400 globular subunits (G-actin), arranged in a double row and
twisted to form a helix. A different type of protein, known as tropomyosin,
lies within the groove between the double row of G-actin monomers. There are
40 to 60 tropomyosin molecules per thin filament, with each tropomyosin spanning
a distance of approximately seven actin subunits.
Attached to the tropomyosin, rather than directly to the actin, is a third type
of protein called troponin. Troponin is actually a complex of three
proteins. These are troponin I (which inhibits the binding of the cross
bridges to actin), troponin T (which binds to tropomyosin), and
troponin C (which binds Ca2+). Troponin and tropomyosin work
together to regulate the attachment of cross bridges to actin, and thus serve as
a switch for muscle contraction and relaxation. In a relaxed muscle, the
position of the tropomyosin in the thin filaments is such that it physically
blocks the cross bridges from bonding to specific attachment sites in the actin.
Thus, in order for the myosin cross bridges to attach to actin, the tropomyosin
must be moved. This requires the interaction of troponin with Ca2+.
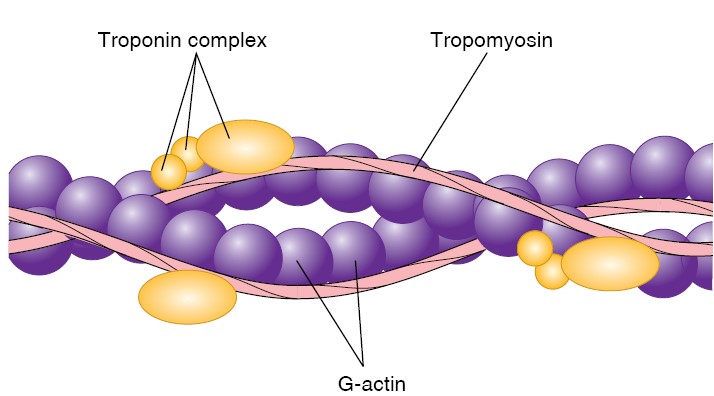
Twitch, Summation, and Tetanus
Contractions of isolated muscles in response to electrical shocks mimic the
behavior of muscles when they contract within the body. When the muscle is
stimulated with a single electric shock of sufficient voltage, it quickly
contracts and relaxes. This response is called a twitch. Increasing the
stimulus voltage increases the strength of the twitch, up to a maximum. The
strength of a muscle contraction can thus be graded, or varied—an obvious
requirement for the proper control of skeletal movements. If a second electric
shock is delivered immediately after the first, it will produce a second twitch
that may partially “ride piggyback” on the first. This response is called
summation. Stimulation of fibers within a muscle in vitro with an
electric stimulator, or in vivo by motor axons, usually results in the
full contraction of the individual fibers. Stronger muscle contractions are
produced by the stimulation of greater numbers of muscle fibers. Skeletal
muscles can thus produce graded contractions, the strength of which
depends on the number of fibers stimulated rather than on the strength of the
contractions of individual muscle fibers. If the stimulator is set to deliver an
increasing frequency of electric shocks automatically, the relaxation time
between successive twitches will get shorter and shorter as the strength of
contraction increases in amplitude. This effect is known as incomplete
tetanus. Finally, at a particular “fusion frequency” of stimulation, there
is no visible relaxation between successive twitches. Contraction is smooth and
sustained, as it is during normal muscle contraction in vivo. This
smooth, sustained contraction is called complete tetanus. The term
tetanus should not be confused with the disease of the same name, which is
accompanied by a painful state of muscle contracture, or tetany.
Muscle Fatigue: If a muscle repeatedly performs
maximal acute or chronic submaximal work, the force of the contractions
gradually decreases. It is called as
muscle fatigue.
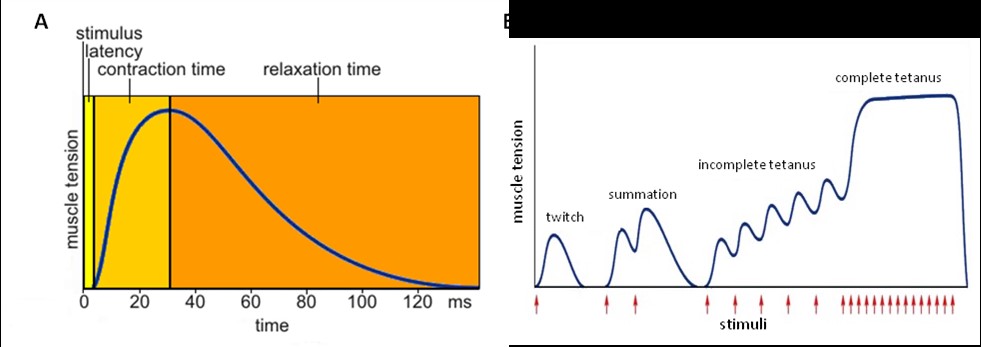
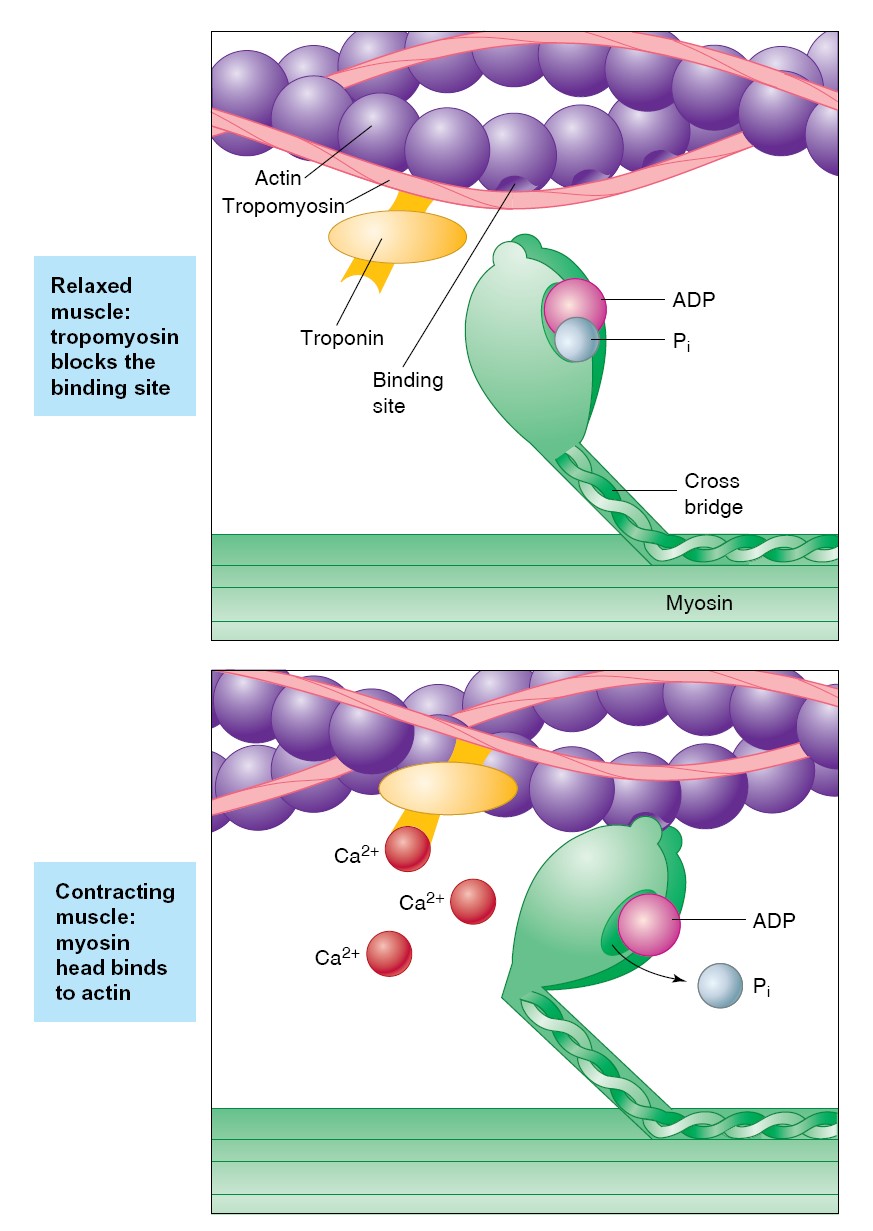
Role of Ca2+
in Muscle Contraction
Scientists long thought that Ca 2 + only served to form the calcium phosphate
crystals that hardened bone, enamel, and dentin. In 1883, Sidney Ringer
published the results of a surprisingly simple experiment that changed that
idea. He isolated rat hearts and found that they beat well when placed in
isotonic solutions made with the hard water from a London tap. When he made the
isotonic solutions with distilled water, however, the hearts gradually stopped
beating. This could be reversed, he found, if he added Ca2+
to the solutions. This demonstrated a role for Ca2+
in muscle contraction, a role that scientists now understand in some detail.
In a relaxed muscle, when tropomyosin blocks the attachment of cross
bridges to actin, the concentration of Ca2+
in the sarcoplasm (cytoplasm of muscle cells) is very low. When the muscle cell
is stimulated to contract, mechanisms that will be discussed shortly cause the
concentration of Ca2+ in the
sarcoplasm to quickly rise. Some of this Ca2+
attaches to troponin, causing a conformational change that moves
the troponin complex and its attached tropomyosin out of the way so that
the cross bridges can attach to actin. Once the attachment sites on the actin
are exposed, the cross bridges can bind to actin, undergo power strokes, and
produce muscle contraction.
The position of the troponin-tropomyosin complexes in the thin filaments is thus
adjustable. When Ca 2 + is not attached to troponin, the tropomyosin is in a
position that inhibits attachment of myosin heads to actin, preventing muscle
contraction. When Ca2+ attaches to
troponin, the troponin-tropomyosin complexes shift position. The myosin heads
can then attach to actin, produce a power stroke, and detach from actin. These
contraction cycles can continue as long as Ca2+
is attached to troponin.
CARDIAC MUSCLE MORPHOLOGY
The
striations in cardiac muscle are similar to those in skeletal muscle, and Z
lines are present. Large numbers of elongated
mitochondria are in close contact with the muscle fibrils. The muscle fibers
branch and interdigitate, but each is a complete unit surrounded by a cell
membrane. Where the end of one muscle fiber abuts on another, the membranes of
both fibers parallel each other through an extensive series of folds. These
areas, which always occur at Z lines, are called intercalated disks. They
provide a strong union between fibers, maintaining cell-to-cell cohesion, so
that the pull of one contractile cell can be transmitted along its axis to the
next. Along the sides of the muscle fibers next to the disks, the cell membranes
of adjacent fibers fuse for considerable distances, forming gap junctions. These
junctions provide low-resistance bridges for the spread of excitation from one
fiber to another. They permit cardiac muscle to function as if it were a
syncytium, even though no protoplasmic bridges are present between cells. The T
system in cardiac muscle is located at the Z lines rather than at the A–I
junction, where it is located in mammalian skeletal muscle.
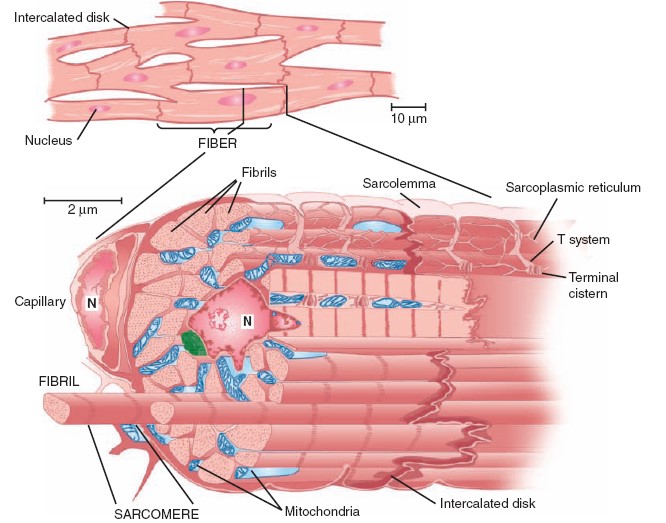
CONTRACTILE RESPONSE
The contractile response of cardiac muscle begins just after the start of
depolarization and lasts about 1.5 times as long as the action potential. The
role of Ca2+ in
excitation—contraction coupling is similar to its role in skeletal muscle.
However, it is the influx of extracellular Ca2+
through the voltage-sensitive DHPR (Dihydropyridine receptor) in
the T system that triggers calcium-induced calcium release through the RyR
(Ryanodine Receptor) at the sarcoplasmic reticulum. Because there is a net
influx of Ca2+
during activation, there is also a more prominent role for plasma
membrane Ca2+ ATPases and the Na+/Ca2+ exchanger in recovery of intracellular
Ca2+ concentrations. During phases 0 to 2 and about half of phase 3 (until the
membrane potential reaches approximately –50 mV during repolarization), cardiac
muscle cannot be excited again; that is, it is in its absolute refractory
period. It remains relatively refractory until phase 4. Therefore, tetanus
of the type seen in skeletal muscle cannot occur. Of course, tetanization of
cardiac muscle for any length of time would have lethal consequences, and in
this sense, the fact that cardiac muscle cannot be tetanized is a safety feature
[Ryanodine drug from Ryania speisosa
which act as insecticide].
SMOOTH
MUSCLE MORPHOLOGY
Smooth muscle is distinguished anatomically from skeletal and cardiac muscle
because it lacks visible cross-striations. Actin and myosin-II are present, and
they slide on each other to produce contraction. However, they are not arranged
in regular arrays, as in skeletal and cardiac muscle, and so the striations are
absent. Instead of Z lines, there are dense bodies in the cytoplasm and
attached to the cell membrane, and these are bound by α-actinin to actin
filaments. Smooth muscle also contains tropomyosin, but troponin appears to be
absent. The isoforms of actin and myosin differ from those in skeletal muscle.
A sarcoplasmic reticulum is present, but it is less extensive than those
observed in skeletal or cardiac muscle. In general, smooth muscles contain few
mitochondria and depend, to a large extent, on glycolysis for their metabolic
needs.
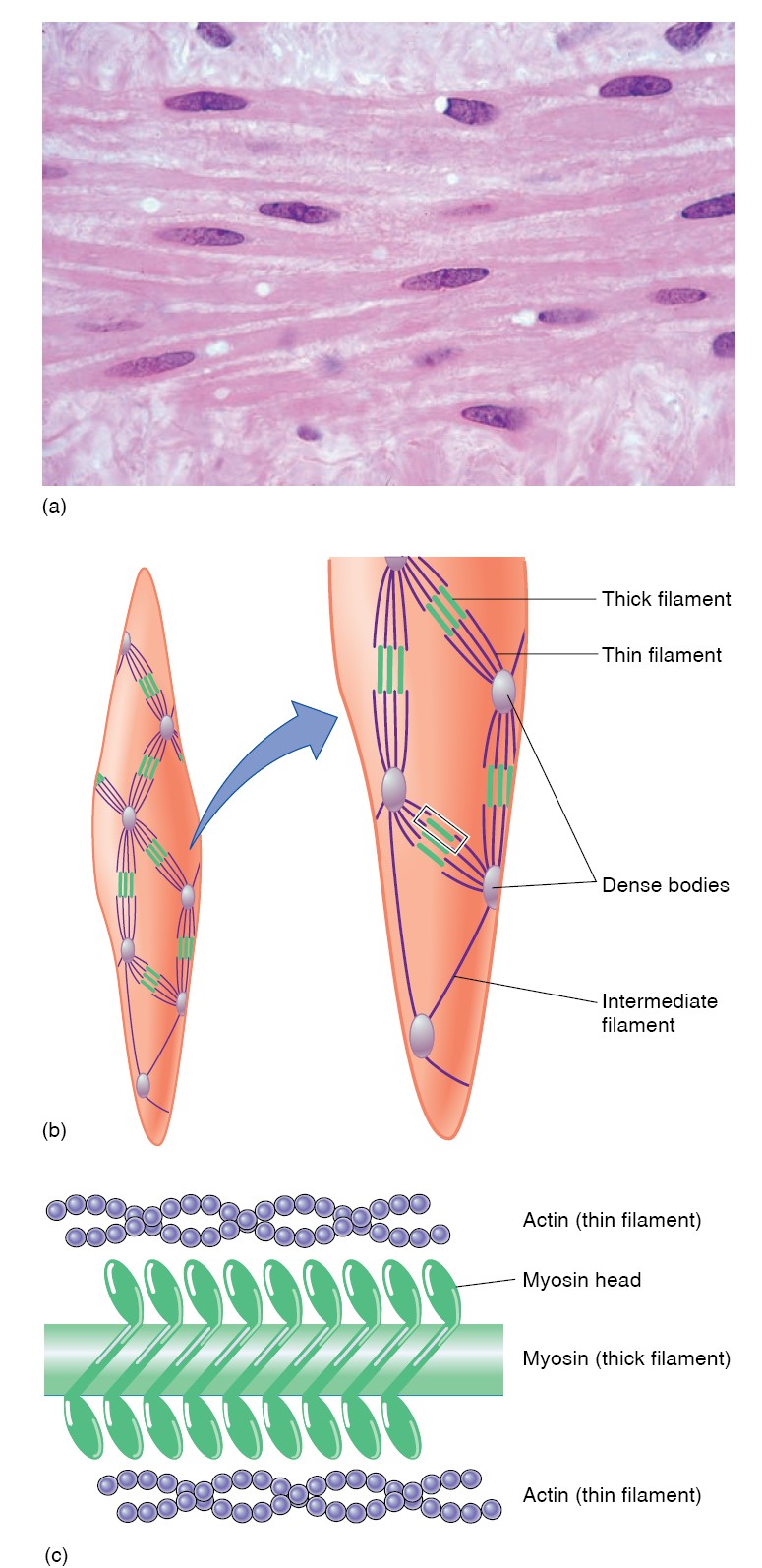
TYPES
There is considerable variation in the structure and function of smooth muscle
in different parts of the body. In general, smooth muscle can be divided into
unitary (or visceral) smooth muscle and multiunit smooth
muscle. Unitary smooth muscle occurs in large sheets, has many
low-resistance gap junctional connections between individual muscle cells, and
functions in a syncytial fashion. Unitary smooth muscle is found primarily in
the walls of hollow viscera. The musculature of the intestine, the uterus, and
the ureters are examples. Multiunit smooth muscle is made up of individual units
with few (or no) gap junctional bridges. It is found in structures such as the
iris of the eye, in which fine, graded contractions occur. It is not under
voluntary control, but it has many functional similarities to skeletal muscle.
Each multiunit smooth muscle cell has en passant endings of nerve fibers, but in
unitary smooth muscle there are en passant junctions on fewer cells, with
excitation spreading to other cells by gap junctions. In addition, these cells
respond to hormones and other circulating substances. Blood vessels have both
unitary and multiunit smooth muscle in their walls.
MOLECULAR BASIS OF CONTRACTION
As in skeletal and cardiac muscle, Ca2+
plays a prominent role in the initiation of contraction of smooth
muscle. However, the source of Ca2+
increase can be quite different in unitary smooth muscle.
Depending on the activating stimulus, Ca2+
increase can be due to influx through voltage- or ligand-gated plasma
membrane channels, efflux from intracellular stores through the RyR, efflux from
intracellular stores through the inositol trisphosphate receptor (IP3R)
Ca2+
channel, or via a combination of these channels. In addition, the lack
of troponin in smooth muscle prevents Ca2+
activation via troponin binding. Rather, myosin in smooth muscle
must be phosphorylated for activation of the myosin ATPase. Phosphorylation and
dephosphorylation of myosin also occur in skeletal muscle, but phosphorylation
is not necessary for activation of the ATPase. In smooth muscle, Ca2+
binds to calmodulin, and the resulting complex activates
calmodulin-dependent myosin light chain kinase. This enzyme catalyzes the
phosphorylation of the myosin light chain on serine at position 19, increasing
its ATPase activity.
Myosin is dephosphorylated by myosin light chain phosphatase in the cell.
However, dephosphorylation of myosin light chain kinase does not necessarily
lead to relaxation of the smooth muscle. Various mechanisms are involved. One
appears to be a latch bridge mechanism by which myosin cross-bridges remain
attached to actin for some time after the cytoplasmic Ca2+ concentration falls.
This produces sustained contraction with little expenditure of energy, which is
especially important in vascular smooth muscle. Relaxation of the muscle
presumably occurs when the Ca2+-calmodulin complex finally dissociates or when
some other mechanism comes into play. The events in multiunit smooth muscle are
generally similar. Unitary smooth
muscle is unique in that, unlike other types of muscle, it contracts when
stretched in the absence of any extrinsic innervation. Stretch is followed by a
decline in membrane potential, an increase in the frequency of spikes, and a
general increase in tone. If
epinephrine or norepinephrine is added to a preparation of intestinal smooth
muscle arranged for recording of intracellular potentials in vitro, the
membrane potential usually becomes larger, the spikes decrease in frequency, and
the muscle relaxes. Acetylcholine has an effect opposite to that of
norepinephrine on the membrane potential and contractile activity of intestinal
smooth muscle.
TYPES OF CONTRACTION
Muscular contraction involves shortening of the contractile elements, but
because muscles have elastic and viscous elements in series with the contractile
mechanism, it is possible for contraction to occur without an appreciable
decrease in the length of the whole muscle. Such a contraction is called
isometric (“same measure” or length). Contraction against a constant load
with a decrease in muscle length is isotonic (“same tension”). Note that
because work is the product of force times distance, isotonic contractions do
work, whereas isometric contractions do not. In other situations, muscle can do
negative work while lengthening against a constant weight.
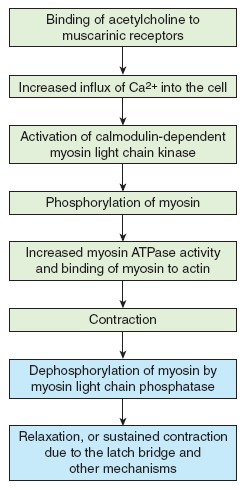
|
Skeletal Muscle |
Cardiac Muscle |
Smooth Muscle |
|
Striated; actin and myosin arranged in sarcomeres |
Striated; actin and myosin arranged in sarcomeres |
Not striated; more actin than myosin; actin inserts into dense bodies
and cell membrane |
|
Well-developed sarcoplasmic reticulum and transverse tubules |
Moderately developed sarcoplasmic reticulum and transverse tubules |
Poorly developed sarcoplasmic reticulum; no transverse tubules |
|
Contains troponin in the thin filaments |
Contains troponin in the thin filaments |
Contains calmodulin, a protein that, when bound to Ca2+, activates the
enzyme myosin light-chain kinase |
|
Ca2+ released into cytoplasm from sarcoplasmic reticulum |
Ca2+ enters cytoplasm from sarcoplasmic reticulum and extracellular
fluid |
Ca2+ enters cytoplasm from extracellular fluid, sarcoplasmic reticulum,
and perhaps mitochondria |
|
Cannot contract without nerve stimulation; denervation results in muscle
atrophy |
Can contract without nerve stimulation; action potentials originate in
pacemaker cells of heart |
Maintains tone in absence of nerve stimulation; visceral smooth muscle
produces pacemaker potentials; denervation results in hypersensitivity
to stimulation |
|
Muscle fibers stimulated independently; no gap junctions |
Gap junctions present as intercalated discs |
Gap junctions generally present |