MICROBIAL METABOLISM
Metabolism refers the sum of biochemical reactions required for energy generation and the use of energy to synthesize cellular materials. The energy generation component is referred as catabolism and the built up of macromolecules and cell organelles are referred as anabolism. During catabolism, the energy is changed from one compound to another and finally conserved as high energy bonds of ATP. ATP is the universal currency for energy. When energy is required for anabolism, it may be sent as high energy bonds of ATP which has the value of 8 kcal per mole.
Based on the source of carbon, the microbes can be divided into two groups namely, autotrophs and heterotrophs. Autotrophs utilize CO2 as sole carbon source and heterotrophs use organic carbon as sole carbon source.
Heterotrophic bacteria, which include all pathogens, obtain energy from oxidation of organic compounds. Carbohydrates (particularly glucose), lipids, and protein are the most commonly oxidized compounds. Biologic oxidation of these organic compounds by bacteria results in synthesis of ATP as the chemical energy source. This process also permits generation of simpler organic compounds (precursor molecules) needed by the bacteria cell for biosynthetic or assimilatory reactions.
The Krebs cycle intermediate compounds serve as precursor molecules (building blocks) for the energy-requiring biosynthesis of complex organic compounds in bacteria. Degradation reactions that simultaneously produce energy and generate precursor molecules for the biosynthesis of new cellular constituents are called amphibolic.
All heterotrophic bacteria require preformed organic compounds. These carbon- and nitrogen-containing compounds are growth substrates, which are used aerobically or anaerobically to generate reducing equivalents (e.g., reduced nicotinamide adenine dinucleotide; NADH + H+); these reducing equivalents in turn are chemical energy sources for all biologic oxidative and fermentative systems. Heterotrophs are the most commonly studied bacteria; they grow readily in media containing carbohydrates, proteins, or other complex nutrients such as blood. Also, growth media may be enriched by the addition of other naturally occurring compounds such as milk (to study lactic acid bacteria) or hydrocarbons (to study hydrocarbon-oxidizing organisms).
Heterotrophic metabolism might be studied under two different headings namely respiration and fermentation.
Glucose is the most common substrate used for studying heterotrophic metabolism. Most aerobic organisms oxidize glucose completely by the following reaction equation:
![]()
This equation expresses the cellular oxidation process called respiration. Respiration occurs within the cells of plants and animals, normally generating 38 ATP molecules (as energy) from the oxidation of 1 molecule of glucose. This yields approximately 380,000 calories (cal) per mode of glucose (ATP ~ 10,000 cal/mole). Thermodynamically, the complete oxidation of one mole of glucose should yield approximately 688,000 cal; the energy that is not conserved biologically as chemical energy (or ATP formation) is liberated as heat (308,000 cal). Thus, the cellular respiratory process is at best about 55% efficient.
Glucose oxidation is the most commonly studied dissimilatory reaction leading to energy production or ATP synthesis. The complete oxidation of glucose may involve three fundamental biochemical pathways. The first is the glycolytic or Embden- Meyerhof-Parnas pathway , the second is the Krebs cycle (also called the citric acid cycle or tricarboxylic acid cycle), and the third is the series of membrane-bound electron transport oxidations coupled to oxidative phosphorylation.
Respiration takes place when any organic compound (usually carbohydrate) is oxidized completely to CO2 and H2O. In aerobic respiration, molecular O2 serves as the terminal acceptor of electrons. For anaerobic respiration, NO3-, SO42-, CO2, or fumarate can serve as terminal electron acceptors (rather than O2), depending on the bacterium studied. The end result of the respiratory process is the complete oxidation of the organic substrate molecule, and the end products formed are primarily CO2 and H2O. Ammonia is formed also if protein (or amino acid) is the substrate oxidized.
Metabolically, bacteria are unlike cyanobacteria (blue-green algae) and eukaryotes in that glucose oxidation may occur by more than one pathway. In bacteria, glycolysis represents one of several pathways by which bacteria can catabolically attack glucose. The glycolytic pathway is most commonly associated with anaerobic or fermentative metabolism in bacteria and yeasts. In bacteria, other minor heterofermentative pathways, such as the phosphoketolase pathway, also exist.
In addition, two other glucose-catabolizing pathways are found in bacteria: the oxidative pentose phosphate pathway (hexose monophosphate shunt), and the Entner-Doudoroff pathway, which is almost exclusively found in obligate aerobic bacteria . The highly oxidative Azotobacter and most Pseudomonas species, for example, utilize the Entner-Doudoroff pathway for glucose catabolism, because these organisms lack the enzyme phosphofructokinase and hence cannot synthesize fructose 1,6-diphosphate, a key intermediate compound in the glycolytic pathway. (Phospho-fructokinase is also sensitive to molecular 02 and does not function in obligate aerobes). Other bacteria, which lack aldolase (which splits fructose-1,6-diphosphate into two triose phosphate compounds), also cannot have a functional glycolytic pathway. Although the Entner-Doudoroff pathway is usually associated with obligate aerobic bacteria, it is present in the facultative anaerobe Zymomonas mobilis (formerly Pseudomonas lindneri). This organism dissimilates glucose to ethanol and represents a major alcoholic fermentation reaction in a bacterium.
If the organism is a respiratory type i.e. complete oxidation of glucose, it needs three essential metabolic components for their respiration and oxidative phosphorylation. They are glycolysis, Citric acid cycle and Electron Transport Chain.
1. GLYCOLYSIS: REFER GLYCOLYSIS NOTES
2. CITRIC ACID CYCLE: REFER TCA NOTES
3. ELECTRON TRANSPORT CHAIN: REFER ETC NOTES
3.1 MICROBIAL ETC:
The final stage of respiration occurs through a series of oxidation-reduction electron transfer reactions that yield the energy to drive oxidative phosphorylation; this in turn produces ATP. The enzymes involved in electron transport and oxidative phosphorylation reside on the bacterial inner (cytoplasmic) membrane. This membrane is invaginated to form structures called respiratory vesicles, lamellar vesicles, or mesosomes, which function as the bacterial equivalent of the eukaryotic mitochondrial membrane.
Respiratory electron transport chains vary greatly among bacteria, and in some organisms are absent. The respiratory electron transport chain of eukaryotic mitochondria oxidizes NADH + H+, NADPH + H+, and succinate (as well as the coacylated fatty acids such as acetyl~SCoA). The bacterial electron transport chain also oxidizes these compounds, but it can also directly oxidize, via non-pyridine nucleotide-dependent pathways, a larger variety of reduced substrates such as lactate, malate, formate, a-glycerophosphate, H2, and glutamate. The respiratory electron carriers in bacterial electron transport systems are more varied than in eukaryotes, and the chain is usually branched at the site(s) reacting with molecular O2. Some electron carriers, such as nonheme iron centers and ubiquinone (coenzyme Q), are common to both the bacterial and mammalian respiratory electron transport chains. In some bacteria, the naphthoquinones or vitamin K may be found with ubiquinone. In still other bacteria, vitamin K serves in the absence of ubiquinone. In mitochondrial respiration, only one cytochrome oxidase component is found (cytochrome a + a3 oxidase). In bacteria there are multiple cytochrome oxidases, including cytochromes a, d, o, and occasionally a + a3.
In bacteria cytochrome oxidases usually occur as combinations of a1: d: o and a + a3: o. Bacteria also possess mixed-function oxidases such as cytochromes P-450 and P-420 and cytochromes c' and c'c', which also react with carbon monoxide. These diverse types of oxygen-reactive cytochromes undoubtedly have evolutionary significance. Bacteria were present before O2 was formed; when O2 became available as a metabolite, bacteria evolved to use it in different ways; this probably accounts for the diversity in bacterial oxygen-reactive hemoproteins.
Cytochrome oxidases in many pathogenic bacteria are studied by the bacterial oxidase reaction, which subdivides Gram-negative organisms into two major groups, oxidase positive and oxidase negative. This oxidase reaction is assayed for by using N,N,N', N'-tetramethyl-p-phenylenediamine oxidation (to Wurster's blue) or by using indophenol blue synthesis (with dimethyl-p-phenylenediamine and a-naphthol). Oxidase-positive bacteria contain integrated (cytochrome c type:oxidase) complexes, the oxidase component most frequently encountered is cytochrome o, and occasionally a + a3. The cytochrome oxidase responsible for the indophenol oxidase reaction complex was isolated from membranes of Azotobacter vinelandii, a bacterium with the highest respiratory rate of any known cell. The cytochrome oxidase was found to be an integrated cytochrome c4:o complex, which was shown to be present in Bacillus species. These Bacillus strains are also highly oxidase positive, and most are found in morphologic group II.
Both bacterial and mammalian electron transfer systems can carry out electron transfer (oxidation) reactions with NADH + H+, NADPH + H+, and succinate. Energy generated from such membrane oxidations is conserved within the membrane and then transferred in a coupled manner to drive the formation of ATP. The electron transfer sequence is accomplished entirely by membrane-bound enzyme systems. As the electrons are transferred by a specific sequence of electron carriers, ATP is synthesized from ADP + inorganic phosphate (Pi) or orthophosphoric acid (H3PO4).
In respiration, the electron transfer reaction is the primary mode of generating energy; electrons (2e-) from a low-redox-potential compound such as NADH + H+ are sequentially transferred to a specific flavoprotein dehydrogenase or oxidoreductase (flavin mononucleotide [FMN] type for NADH or flavin adenine dinucleotide [FAD] type for succinate); this electron pair is then transferred to a nonheme iron center (FeS) and finally to a specific ubiquinone or a naphthoquinone derivative. This transfer of electrons causes a differential chemical redox potential change so that within the membrane enough chemical energy is conserved to be transferred by a coupling mechanism to a high-energy compound (e.g., ADP + Pi Æ ATP). ATP molecules represent the final stable high-energy intermediate compound formed.
A similar series of redox changes also occurs between ubiquinone and cytochrome c, but with a greater differential in the oxidation-reduction potential level, which allows for another ATP synthesis step. The final electron transfer reaction occurs at the cytochrome oxidase level between reduced cyotchrome c and molecular O2; this reaction is the terminal ATP synthesis step.
In aerobic organisms, the terminal electron acceptor will be of O2. In some anaerobic organisms, after the electron transport chain, instead of O2, some inorganic compounds like sulphate, nitrate or some organic compounds like fumarate act as terminal acceptor. Such type of respiration is referred as anaerobic respiration and the normal O2 mediated respiration is referred as aerobic respiration. Some terminal electron acceptor shown in the table below:
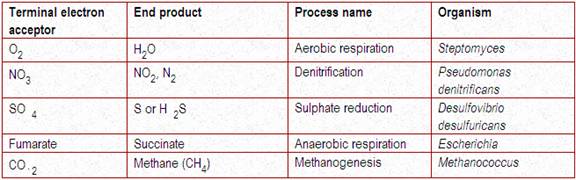
Fermentation, another example of heterotrophic metabolism, requires an organic compound as a terminal electron (or hydrogen) acceptor. In fermentations, simple organic end products are formed from the anaerobic dissimilation of glucose (or some other compound). Energy (ATP) is generated through the dehydrogenation reactions that occur as glucose is broken down enzymatically. The simple organic end products formed from this incomplete biologic oxidation process also serve as final electron and hydrogen acceptors. On reduction, these organic end products are secreted into the medium as waste metabolites (usually alcohol or acid). The organic substrate compounds are incompletely oxidized by bacteria, yet yield sufficient energy for microbial growth. Glucose is the most common hexose used to study fermentation reactions.
In the late 1850s, Pasteur demonstrated that fermentation is a vital process associated with the growth of specific microorganisms, and that each type of fermentation can be defined by the principal organic end product formed (lactic acid, ethanol, acetic acid, or butyric acid). His studies on butyric acid fermentation led directly to the discovery of anaerobic microorganisms. Pasteur concluded that oxygen inhibited the microorganisms responsible for butyric acid fermentation because both bacterial mobility and butyric acid formation ceased when air was bubbled into the fermentation mixture. Pasteur also introduced the terms aerobic and anaerobic. His views on fermentation are made clear from his microbiologic studies on the production of beer (from Etudes sur la Biere, 1876):
In the experiments which we have described, fermentation by yeast is seen to be the direct consequence of the processes of nutrition, assimilation and life, when these are carried on without the agency of free oxygen. The heat required in the accomplishment of that work must necessarily have been borrowed from the decomposition of the fermentation matter.... Fermentation by yeast appears, therefore, to be essentially connected with the property possessed by this minute cellular plant of performing its respiratory functions, somehow or other, with the oxygen existing combined in sugar.
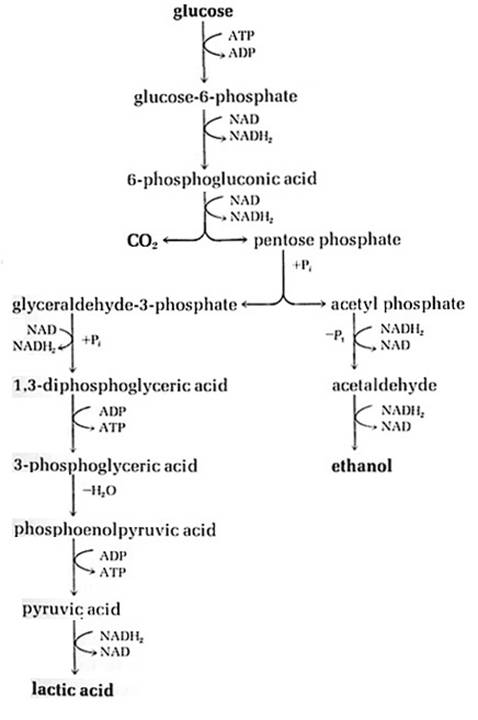
For most microbial fermentations, glucose dissimilation occurs through the glycolytic pathway. The simple organic compound most commonly generated is pyruvate, or a compound derived enzymatically from pyruvate, such as acetaldehyde, a-acetolactate, acetyl ~ SCoA, or lactyl ~ SCoA. Acetaldehyde can then be reduced by NADH + H+ to ethanol, which is excreted by the cell. The end product of lactic acid fermentation, which occurs in streptococci (e.g., Streptococcus lactis) and many lactobacilli (e.g., Lactobacillus casei, L pentosus), is a single organic acid, lactic acid. Organisms that produce only lactic acid from glucose fermentation are homofermenters. Homofermentative lactic acid bacteria dissimilate glucose exclusively through the glycolytic pathway. Organisms that ferment glucose to multiple end products, such as acetic acid, ethanol, formic acid, and CO2, are referred to as heterofermenters.
Examples of heterofermentative bacteria include Lactobacillus, Leuconostoc, and Microbacterium species. Heterofermentative fermentations are more common among bacteria, as in the mixed-acid fermentations carried out by bacteria of the family Enterobacteriaceae (e.g., Escherichia coli, Salmonella, Shigella, and Proteus species). Many of these glucose fermenters usually produce CO2 and H2 with different combinations of acid end products (formate, acetate, lactate, and succinate). Other bacteria such as Enterobacter aerogenes, Aeromonas, Serratia, Erwinia, and Bacillus species also form CO2 and H2 as well as other neutral end products (ethanol, acetylmethylcarbinol [acetoin], and 2,3-butylene glycol). Many obligately anaerobic clostridia (e.g., Clostridium saccharobutyricum, C thermosaccharolyticum) and Butyribacterium species ferment glucose with the production of butyrate, acetate, CO2, and H2, whereas other Clostridum species (C acetobutylicum and C butyricum) also form these fermentation end products plus others (butanol, acetone, isopropanol, formate, and ethanol). Similarly, the anaerobic propionic acid bacteria (Propionibacterium species) and the related Veillonella species ferment glucose to form CO2, propionate, acetate, and succinate. In these bacteria, propionate is formed by the partial reversal of the Krebs cycle reactions and involves a CO2 fixation by pyruvate (the Wood-Werkman reaction) that forms oxaloacetate (a four-carbon intermediate). Oxaloacetate is then reduced to malate, fumarate, and succinate, which is decarboxylated to propionate. Propionate is also formed by another three-carbon pathway in C propionicum, Bacteroides ruminicola, and Peptostreptococcus species, involving a lactyl ~ SCoA intermediate. The obligately aerobic acetic acid bacteria (Acetobacter and the related Gluconobacter species) can also ferment glucose, producing acetate and gluconate.
For thermodynamic reasons, bacteria that rely on fermentative process for growth cannot generate as much energy as respiring cells. In respiration, 38 ATP molecules (or approximately 380,000 cal/mole) can be generated as biologically useful energy from the complete oxidation of 1 molecule of glucose (assuming 1 NAD(P)H = 3 ATP and 1 ATP ADP + Pi = 10,000 cal/mole). Table 4-3 shows comparable bioenergetic parameters for the lactate and ethanolic fermentations by the glycolytic pathway. Although only 2 ATP molecules are generated by this glycolytic pathway, this is apparently enough energy to permit anaerobic growth of lactic acid bacteria and the ethanolic fermenting yeast, Saccharomyces cerevisiae. The ATP-synthesizing reactions in the glycolytic pathway specifically involve the substrate phosphorylation reactions catalyzed by phosphoglycerokinase and pyruvic kinase. Although all the ATP molecules available for fermentative growth are believed to be generated by these substrate phosphorylation reactions, some energy equivalents are also generated by proton extrusion reactions (acid liberation), which occur with intact membrane systems and involve the proton extrusion reactions of energy conservation as it applies to fermentative metabolism.
The Glycolytic pathway(EMP), phoshoketolase pathway and Entner Doudoroff pathway end with one or two ATP synthesis by substrate level phosphoroylation. There won’t be any external source of electron acceptor will come in these reactions.
1. Glycolysis:
This is the most familiar and common to most of the organism. The pathway is operated by yeast to produce alcohol and lactic acid bacterial to produce lactic acid and several organic acids, gases, fatty acids and alcohols.
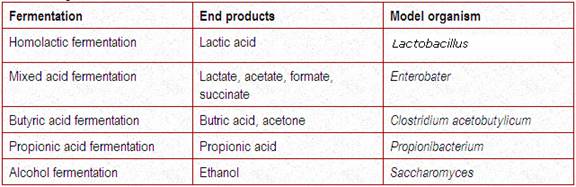
2. Phosphoketolase Pathway ( Heterolactic pathway):
The phosphoketolase pathway is distinguished by the key cleavage enzyme phosphoetolase, which cleaves pentose to glyceraldehydes – 3 – phosphate and acetyl phosphate. The pathway ends with ethanol and lactic acid. Ex. Lactobacillus, Leconostoc. The pentose molecules for this pathway might be derived from HMP shut also.
Glucose ----à 1 lactate + 1 ethanol + 1 CO2 + ATP
This pathway is useful in the dairy industry for preparation of Kefir ( fermented milk ), yogurt, etc.,
3. Entner Doudoroff Pathway:
Only few bacteria like, Zymomonas mobilis employ the ED pathway. REFER ED NOTES
II. AUTOTROPHIC METABOLISM:
Autotrophs use CO2 as their sole carbon source. There are two types such as photoautotrophs and chemoautotrophs. Photoautotrophs use light as energy source and CO2 as carbon source. Chemoautotrophs use chemicals as energy source and CO2 as carbon source.
II.1. PHOTOTROPHS:
Phototrophs used sunlight to produce ATP through phosphorylation referred as photophosphorylation. The phototrophs convert the light energy to chemical energy (ATP) through the process called photosynthesis.
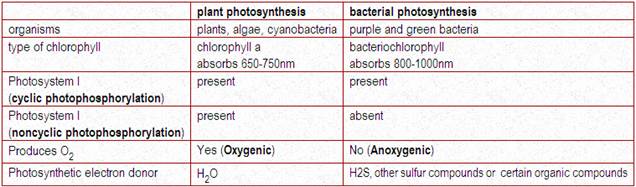
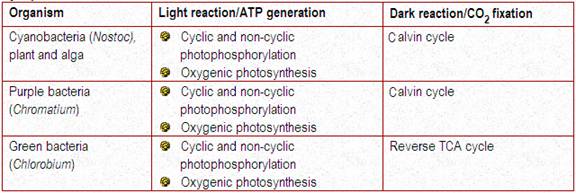
Many prokaryotes (bacteria and cyanobacteria) possess phototrophic modes of metabolism (Table 4-1) . The types of photosynthesis in the two groups of prokaryotes differ mainly in the type of compound that serves as the hydrogen donor in the reduction of CO2 to glucose (Table 4-1). Phototrophic organisms differ from heterotrophic organisms in that they utilize the glucose synthesized intracellularly for biosynthetic purposes (as in starch synthesis) or for energy production, which usually occurs through cellular respiration.
A new photosynthetic, and nitrogen fixing bacterium, Heliobacterium chlorum, staining Gram positive was isolated, characterized, and found to contain a new type of chlorophyll, i.e., bacteriochlorophyll 'g'. 16S r-RNA sequence analyses showed this organism to be phylogenetically related to members of the family Bacillaceae, although all currently known phototrophes are Gram negative (see Table 4.4). A few Heliobacteriium strains did show the presence of endospores. Another unusual phototrophe is the Gram negative Halobacterium halobium (now named Halobacterium salinarium), an archaebacterium growing best at 30°C in 4.0-5.0 M (or 25%, w/v) NaCl. This bacterium is a facultative phototrophe having a respiratory mode; it also possesses a purple membrane within which bacteriorhodopsin serves as the active photosynthetic pigment. This purple membranae possesses a light driven proton translocation pump which mediates photosynthetic ATP synthesis via a proton extrusion reaction (see Mitchell Hypothesis).
Photosynthesis is a type of metabolism in which catabolism and anabolism occur as sequence. The catabolic reaction of photosynthesis is light reaction in which the light energy is converted to chemical energy (ATP) and electrons or reducing powers (NADPH) . The anabolic reaction of photosynthesis is dark reaction in which CO2 is converted to organic molecules ( carbohydrates ), which is also called as CO2 fixation.
For conversion of light energy to ATP, the bacterial pocess light harvesting pigments. They are chlorophyll a, carotenoids, phycobiliproteins which are present in cyanobacteria and bacteriochlorophyll which are present in purple sulphur bacteris. In bacteria there are two types of light reactions namely Cyclic and Non-cyclic photophosphorylation reactions and two types of CO2 fixation namely calvin cycle and reverse TCA cycle occur.
In plant and cyanobacteria, both cyclic and non-cyclic photophosphorylation occur whereas in purple bacteria, the cyclic photophosphorylation only occurs. In plant and cyanobacteria, the electron source is water, by photolysis, it split into H+ and O2 and during the process, O2 is evolved and referred as oxygenic photosynthesis. Since sulphur bacteria is an anaerobic bacterium, they use H2S instead of H2O as electron donor. Since, there won’t be any O2 evolution during photosynthesis, referred as anoxygenic photosynthesis.
Light reaction:
1. Cyclic photophosphorylation: REFER PHOTOSYNTHESIS NOTES
2. Non-cyclic photophosphorylation: REFER PHOTOSYNTHESIS NOTES
3. Microbial cyclic photophosphorylation:
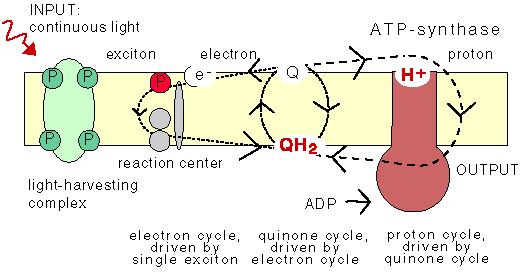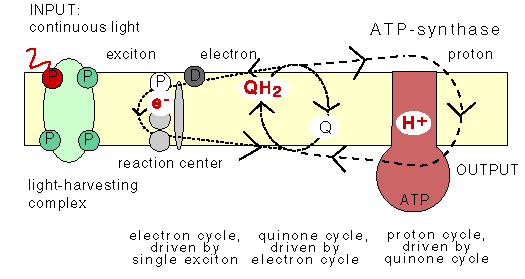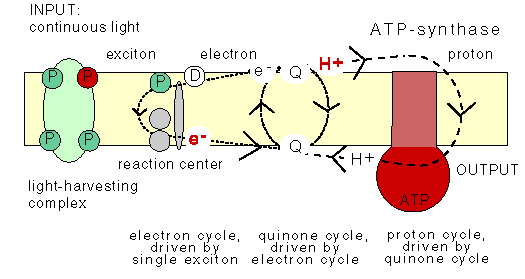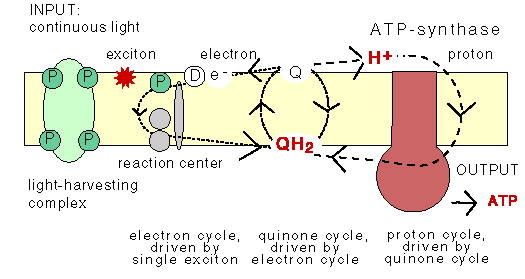
Dark reaction:
1. Calvin cycle: REFER PHOTOSYNTHESIS NOTES
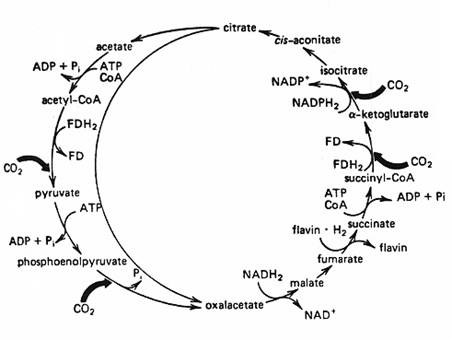
2. Reverse TCA cycle:
Photosynthetic green bacteria ( Chlorobium ) derive NADPH and ATP through cyclic phosphorylation, but CO2 fixation is by reverse TCA cycle. Since TCA cycle is amphibolic pathway, it can also be used to fix the CO2 if operated reversely.
Another way of CO2 fixation is by methanogens. They uses CO2 as terminal electron acceptor and forms methane. They also fix by acetyl CoA pathway for fixing CO2.
CO2 fixation by methanogens (CODH Pathway):
The methanogens, a very abundant group of procaryotes, use CO2 as a source of carbon for growth, and as a final electron acceptor in an energy-producing process that produces methane. If a methanogen is fed labeled CO2 as a sole form of carbon, 95 percent of the label winds up in methane and 5 percent winds up in cell material. The methanogens fix CO2 by means of the enzyme CODH (carbon monoxide dehydrogenase) and the Acetyl CoA pathway.
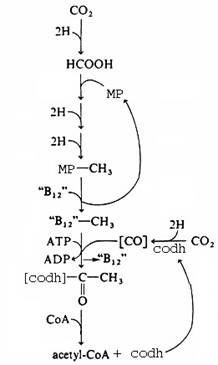
The pathway of methanogenesis steadily reduces CO2 to the methyl (CH3) level, mediated by the coenzyme methanopterin (MP), related to folic acid. MP-CH3 may be reduced to methane (not shown) or the MP may be replaced by a vitamin B12-like molecule to enter the pathway of CO2 fixation. The "B12"-CH3 is substrate for CO fixation mediated by the CODH. CODH reduces CO2 to CO and adds the CO to "B12"-CH3 to form acetyl-[CODH]. Coenzyme A (CoA) then replaces the CODH, resulting in the formation of Acetyl CoA, which is in the heart of biosynthetic metabolism. The net effect is the reduction of 2 CO2 to Acetyl CoA.
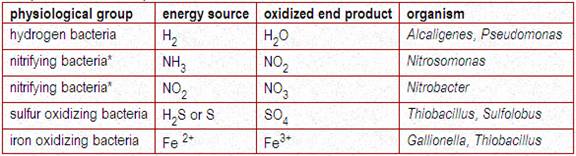
II.2. CHEMOAUTOTROPHS:
Since the chemoautotrophs use inorganic chemicals for their energy and electron source, they are referred as chemolithotrophs or chemolithotrophic autotrophs. These organisms remove electron from an inorganic substance and put them through electron transport chain for ATP synthesis. At the same time, the electrons were also flow though reverse electron transport chain and with the end product of NADPH. These ATP and NADPH were used for CO2 fixation through calvin cycle. These bacteria are obligate aerobic organisms. Thiobacillus ferrooxidans, which gets its energy for autotrophic growth by oxidizing elemental sulfur or ferrous iron, and T denitrificans, which gets its energy by oxidizing S2O3 anaerobically, using NO3- as the sole terminal electron acceptor. T denitrificans reduces NO3 to molecular N2, which is liberated as a gas; this biologic process is called denitrification.
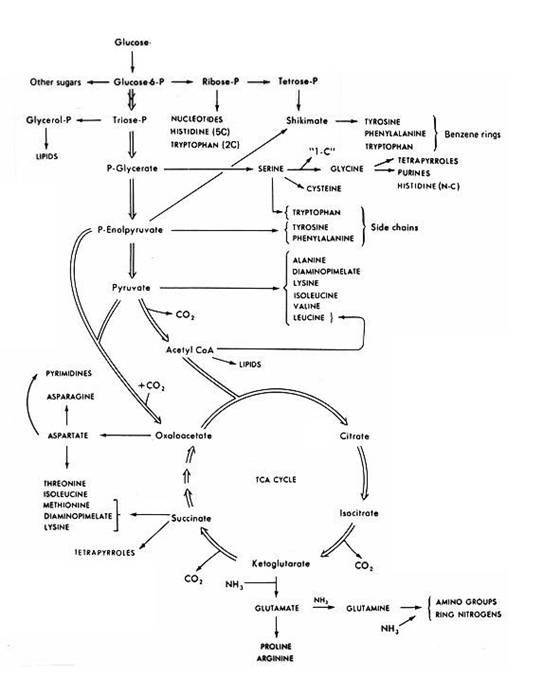
III. Biosynthetic pathways in microbes: