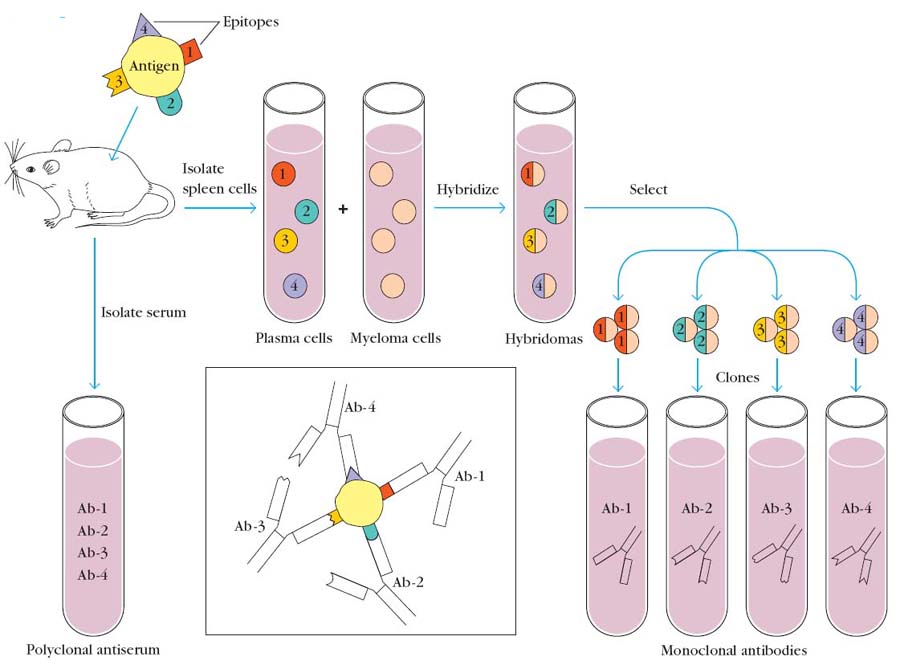
MONOCLONAL ANTIBODY PRODUCTION
Antibodies having single specificity produced from a single clone of B cell are referred as Mono clonal antibodies (MAbs). In 1975, Georges Köhler and Cesar Milstein devised a method for preparing monoclonal antibody, which quickly became one of immunology’s key technologies. The significance of the work by Köhler and Milstein was acknowledged when each was awarded a Nobel Prize.
CONVENTIONAL METHOD OF ANTIBODY PRODUCTION:
Group of antibodies having different specificity produced from different clone of B cell is referred as polyclonal antibodies. Antigens with multi-epitopes injected into mice. After an incubation period serum is isolated from mice. It will contain antibodies against different epitopes and they are also produced from different clone of cells. Serums with antibodies are known as antiserum which is produced against an injected antigen like antitoxin which is nothing but antibody produced against toxins.
Antigens with multivalent epitope injected into mice. After incubation period B cells are isolated. They are hybridized with myeloma cells with the help of fusogens different types of B cells are separated. Antibodies from each type act as mono clonal antibodies.

Monoclonal antibodies are produced mainly by hybridoma technology.
HYBRIDOMA TECHNOLOGY:
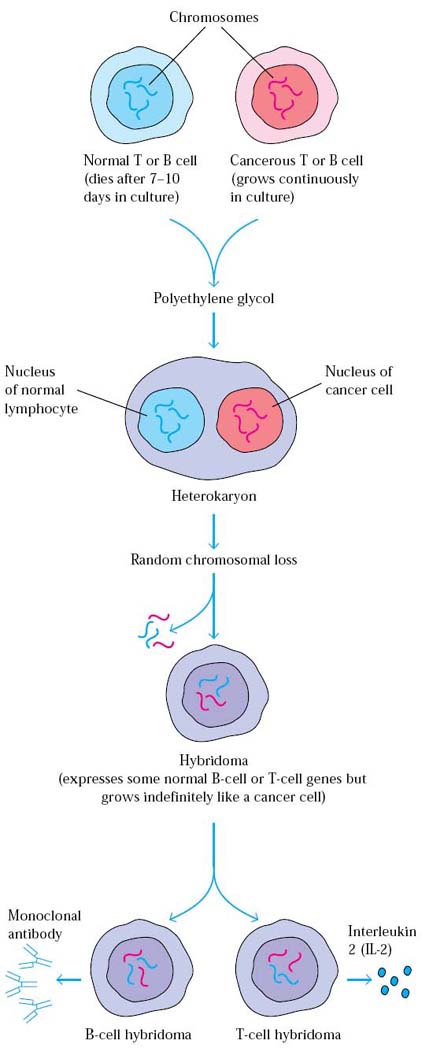
It refers to the production of hybridoma cells by a technology. In this technology, cells of interest hybridized with cancer cells. The aim of this technology is to provide immortality to the cells of interest.
When cells of interest fused with cancer cells with the help of fusogens like sendaivirus or polyethylene glycol (PEG), first cytoplasmic membrane of two cells are fused to form heterokaryon i.e. cells with two different nucleuses. Then the two nucleuses combined to form cybrid or hybrid cells. Hybrid cells contain combination of both cell nuclear materials but maintaining species usual chromosomal number by loss of extra chromosome. In cybrid cells genetic material of either of the cell maintained completely and the other one is lost completely i.e. cybrid cells are hybrids considering the cytoplasmic component.
Of the three possibility of cells like unfused cells, hybridoma cells and cybrid cells, hybridoma cells selected by using HAT (Hypoxanthine Aminopterin Thymidine) medium.
HAT MEDIUM SELECTION:
HAT selection depends on the fact that mammalian cells can synthesize nucleotides by two different pathways: the de novo and the salvage pathways. The de novo pathway in which a methyl or formyl group is transferred from an activated from of tetrahydrofolate, is blocked by Aminopterin, a folic acid analog. When the de novo pathway is blocked, cells utilize the salvage pathway, which bypasses the aminopterin block by converting purines and pyrimidines directly into DNA. The enzymes catalyzing the salvage pathway include hypoxanthine-guanine phosphorribosyl transferase (HGPRT) and thymidine kinase (TK). A mutation in either of these two enzymes blocks the salvage pathway. HAT medium contains Aminopterin to block the de novo pathway and hypoxanthine and thymidine to allow growth via the salvage pathway. When two types of cells, one with a mutation in TK and the other with a mutation in HGPRT are fused, only the hybrid cells will contain the full complement of necessary enzymes for growth on HAT medium via the salvage pathway. Thus only hybrid cells will grow in HAT medium.
MONOCLONAL ANTIBODY PRODUCTION BY HYBRIDOMA TECHNOLGY:
Monoclonal antibodies produced in the following steps:
1. Preparation of Cells:
For the production of monoclonal antibodies, cells should be properly prepared. B cell and myeloma cells are used for monoclonal antibody production. Genetic machinery of B cells should be modified as Ig+, HGPRT--- and TK+. This is achieved through site directed mutagenesis process. These B cells are actually isolated from mice to which antigen of our interest is injected and it also posses the ability of producing antibody against antigen of our interest. Genetic machinery of myeloma cells are modified as Ig---, HGPRT+ and TK---. The reason for genetic modification is to provide antibody producing ability to B cells and for selecting hybrid cell from the culture after hybridization. Myeloma cells are used to provide immortality property.
2. Fusion of Cells:
After preparing B cell and myeloma cell, they are fused with the help of sendaivirus or polyethylene glycol (PEG). These fusogens fuse the cells to produce fused B cells-myeloma cells, cybrid and hybrids. Some unfused cells may also present in the medium.
3. Selection of Hybrid cells:
After fusing the cells with the help of fusogens they are placed in a culture medium with selection medium of HAT. Aminopterin inhibits de novo nucleotide biosynthesis. When de novo synthesis inhibited cells can use salvage pathway to produce nucleotides for that they require HGPRT and TK. Since one of this enzyme is absent in B cells, myeloma cells and cybrids, they are unable to survive in the selection medium. Those hybrid cells which are having functional HGPRT and TK are found to be capable of growing in selection medium. It can be otherwise called as that they are selected in selection.
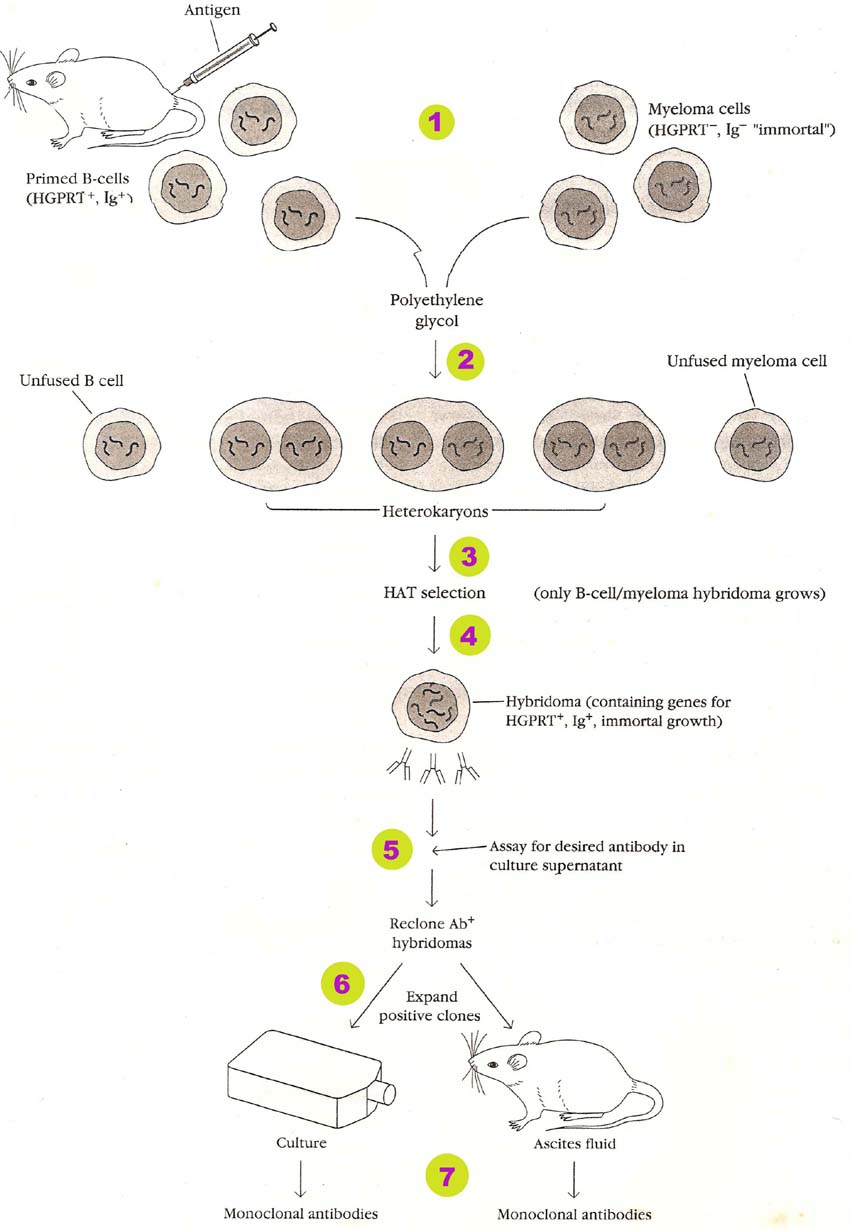
4. Culturing of selected hybridoma cells:
After hybridoma cells are selected, they are cultured in microtiter wells in such a way that each well consists of single hybridoma cells. So that, mono clone is produced i.e. Group of cells derived from single hybridoma cells.
5. Screening cells for antibody production:
Eventhough hybridoma cells survived in the selection medium and they also form clones, there ability to form antibody is not tested yet. This property is tested using ELISA or RIA tests. Those clones which have the ability to produce antibody of our interest are allowed for next step of monoclonal antibody production.
6. Propagation of screened clones:
It is nothing but culturing or allowing selected clone cells to grow in invitro or invivo conditions respectively. In invitro technique clone cells are cultured in tissue culture flask with suitable medium. The production rate is found to be 10 – 100micrograms per ml. In invivo method, selected clones are injected into the peritoneal cavity of histocompatible mice and allowed to multiply and produce antibody. The rate of antibody production is 25 milligram per ml.
7. Isolation of antibodies:
In either of above methods, samples are collected and from these monoclonal antibodies are separated by affinity chromatography and they are used for different studies.
TYPES OF MONOCLONAL ANTIBODIES:
Following are the different types of monoclonal antibodies:
A. Immunotoxins:
It is nothing but the complex of antibodies and toxins like ricin, diphtheria toxin etc., i.e. toxins linked to immunoglobins at their Fc region. Immunotoxins are mainly used against tumors. When Immunotoxins for a particular tumor cells injected, they reached the target cell with the help of antibodies. After their attachment to tumor cells, it is taken up by tumor cells through endocytosis. After this, cellular metabolism interfered with the help of toxins and cells died. Because of the use of monoclonal antibodies, toxins are directed towards specific tumor cells.
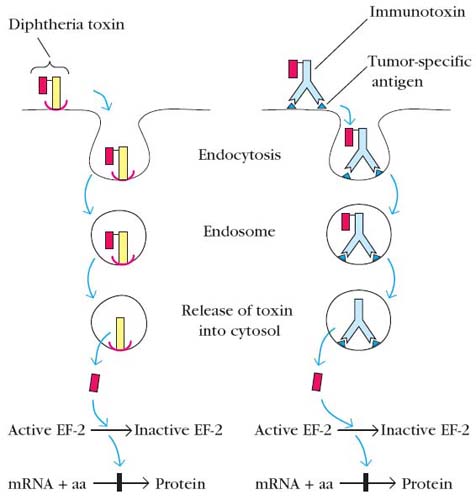
B. Chimeric Immunotoxins:
The term chimera used because unlike Immunotoxins, toxin replaces one of the constant region domain of Ig especially carboxy terminal domain of H chain. It can also be used to treat cancers. It is advantageous than Immunotoxins in the sense that it won’t activate complements and consequent inflammatory reaction after binding to the antigens. This is because of the lack of Fc region in chimeric Immunotoxins.
C. Humanized antibodies:
When antibodies from mice injected as anti-tumor agents, they can stimulate immunogenic reactions against them in the host. This can be avoided to certain extent using humanized antibodies. Humanized antibodies contain constant region of both L and H chain derived from humans and variable regions derived from mice. It is named so because of the presence of human gene coding regions. It can be used as diagnostic tool also.
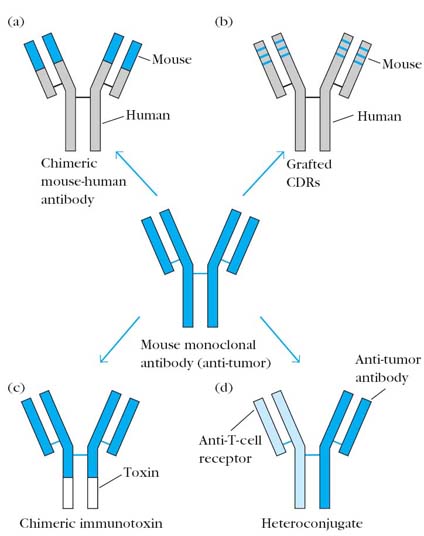
D. CDR grafted Antibodies:
It is the modified form of humanized antibodies i.e. hypervariable region of both light and heavy chains variable region gene only derived from mice. The remaining genes derived from human. It is used against cancer and it also act as immunosuppressor. Usually in such cases, antibodies are directed against CD3 or CD4 components. Because once these components blocked, activation of cell mediated immunity affected.
E. Heterconjugate antibodies:
The word hetero means that antibodies with different specificity i.e. different Paratopes having different specificity in single antibody. This type of antibody specifically used to treat cancer. In this case, one paratope of antibody directed against tumor cells whereas other paratope is directed against cytotoxic cells usually Tc cells. These antibodies bring effective cytotoxic cells close to tumor cells, so that they are easily lysed by cytotoxic mechanisms.
ABZYMES:
Antibodies with enzymatic activity are referred as abzymes. They are otherwise called as catalytic antibodies. The binding of an antibody to its antigen is similar in many ways to the binding of an enzyme to its substrate. In both cases the binding involves weak, non-covalent interactions and exhibits high specificity and often high affinity. What distinguishes an antibody-antigen interaction from an enzyme- substrate interaction is that the antibody does not alter the antigen, whereas the enzyme catalyzes a chemical change in its substrate. However, like enzymes, antibodies of appropriate specificity can stabilize the transition state of a bound substrate, thus reducing the activation energy for chemical modification of the substrate. The similarities between antigen-antibody interactions and enzyme-substrate interactions raised the question of whether some antibodies could behave like enzymes and catalyze chemical reactions. To investigate this possibility, a hapten-carrier complex was synthesized in which the hapten structurally resembled the transition state of an ester undergoing hydrolysis. Spleen cells from mice immunized with this transition state analogue were fused with myeloma cells to generate monoclonal anti-hapten monoclonal antibodies. When these monoclonal antibodies were incubated with an ester substrate, some of them accelerated hydrolysis by about 1000-fold; that is, they acted like the enzyme that normally catalyzes the substrate’s hydrolysis. The catalytic activity of these antibodies was highly specific; that is, they hydrolyzed only esters whose transition-state structure closely resembled the transition state analogue used as a hapten in the immunizing conjugate.
IG GENE LIBRARIES CONSTRUCTION:
A quite different approach for generating monoclonal antibodies employs the polymerase chain reaction (PCR) to amplify the DNA encoding antibody heavy chain and light chain Fab fragments from hybridoma cells. A promoter region and EcoRI site are added to the amplified sequences and the resulting constructs are inserted into bacteriophage lambda, yielding separate heavy and light chain libraries. Cleavage with EcoRI and random joining of the heavy and light chain genes yield numerous novel heavy lights constructs. This procedure generates enormous diversity of antibody specificities; clones containing these random combinations of H + L chains can be rapidly screened for those secreting antibody to a particular antigen.
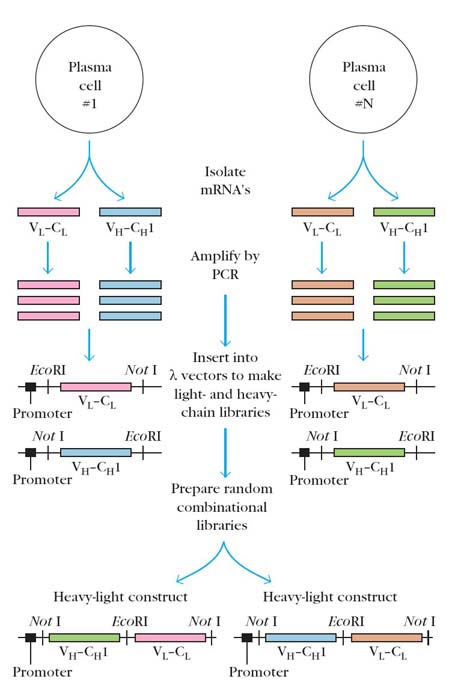
For example, in one study a million clones were screened in just 2 days, with over 100 clones being identified that produced antibody specific for the desired antigen. The technique has the potential of producing an enormous repertoire of antibody specificities without the limitations of antigen priming and hybridoma technology that currently complicate the production of monoclonal antibodies.
APPLICATIONS OF MONOCLONAL ANTIBODIES:
Monoclonal antibodies are proving to be very useful as diagnostic, imaging, and therapeutic reagents in clinical medicine. Initially, monoclonal antibodies were used primarily as in vitro diagnostic reagents. Among the many monoclonal antibody diagnostic reagents now available are products for detecting pregnancy, diagnosing numerous pathogenic microorganisms, measuring the blood levels of various drugs, matching histocompatibility antigens, and detecting antigens shed by certain tumors. Radiolabeled monoclonal antibodies can also be used in vivo for detecting or locating tumor antigens, permitting earlier diagnosis of some primary or metastatic tumors in patients. For example, monoclonal antibody to breast-cancer cells is labeled with iodine-131 and introduced into the blood to detect the spread of a tumor to regional lymph nodes. This monoclonal imaging technique can reveal breast-cancer metastases that would be undetected by other, less sensitive scanning techniques.
Mainly monoclonal antibodies applied in two fields namely,
I. Diagnostic Field
II. Therapeutic Field
I. Diagnostic Field:
For the following purposes, monoclonal antibodies used:
In the above mentioned conditions, the basic principle is production of antibodies against antigen and identification or quantification of antigen and antibody complex.
II. Therapeutic Field:
There are four different headings available in this field namely,
a. Anti-tumor therapy:
In anti-tumor therapy, antibodies against tumor antigen produced and they are converted into either Immunotoxins, or chimeric Immunotoxins or Heterconjugate antibodies. When these antibodies utilized, they damage tumor cells and tumor growth controlled. These anti-tumor antibodies are called as “magic bullets”.
b. Immunosuppression:
During transplantation between partially incompatible individuals, host versus graft rejections are suppressed using monoclonal antibodies against TCR, BCR, Co-receptor complex and cytokines etc., Hypersensitivity reactions are also treated with blocking monoclonal antibodies.
c. Fertility control:
By producing antibodies against HCG or trophoblast, fertility controlled.
d. Drug toxicity reversal:
Toxicity produced by drugs is treated using monoclonal antibodies against drugs, so that the functions of drugs are blocked and effect reversed.