LIPIDS
Lipids are found to be water insoluble but they are found to be soluble in fat solvent. They are heterogeneous group of fatty acids. They include fats, oils, waxes and other related substances. They are oily or greasy substances. Thus they are hydrophobic in nature. Proteins, polysaccharides, DNA and RNA are macromolecules. Lipids are not generally classed as macromolecules, even though they share some features of macromolecules. For example: lipids synthesized as linear polymers and they self assemble into larger structures.According to Bloor, lipids are compounds having the following characteristics:
Lipids are isolated from tissues using chloroform-methanol (2:1) solvent mixture. Care should be taken while isolating lipids because unsaturated fatty acids present in tissues are easily oxidized or become polymerized and isomerizes. From the extract, the various classes of lipids may be separated by chromatographic procedures like column, thin layer, paper and gas chromatography techniques. The lipid content of tissues may vary widely. Rapidly growing embryonic tissue may have as little as 1 to 2 percent total lipid, while tissues in which there is storage may contain 90% lipid.
BIOLOGICAL IMPORTANCE OF LIPIDS:
ENERGY SOURCE: Lipids act as fuel in the body. Lipids are found to be superior to carbohydrates and protein in providing energy to body with respect to their energy released by their oxidation. Lipids yield 9.5Kcal/gram whereas proteins and carbohydrates yield only about 4Kcal/gram.
LIPID STORAGE: Since lipids are insoluble in aqueous solution, they can be stored easily in body. They can be stored in the body in almost unlimited amount in contrast to carbohydrates which is stored in limited amount and places.
INSULATION: Fats are usually stored in the subcutaneous layers. They exert insulating property. This is found to be useful especially useful in those animals living in the cold climate like whale etc., Lipids stored around internal organs found to be padding and protecting the organs.
STRUCTURAL ROLE: Lipids play a vital role in structure by being the important component of lipid bilayer in membrane. Especially phospholipids and cholesterols are important.
ENDOCRINE FUNCTION: Hormones like adrenocorticoids, mineralocorticoids, sex hormones and vitamin D were found to be synthesized from lipids like cholesterol.
VITAMIN ABSORPTION: Lipids are essential for the absorption of fat soluble vitamins like vitamin A, D, E and K.
NERVOUS SYSTEM: Lipids are found to be important constituent of the nervous system because nerve cells found to contain greater amount of lipids they play vital role nerve cell insulation and impulse conduction.
ANTIBIOTIC ACTIVITY: Squalamine, a steroid from Sharks' blood, has been found to be antifungal and antibacterial activity.
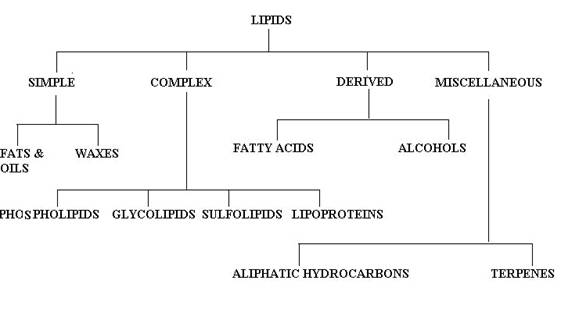
CLASSIFICATION OF LIPIDS:
There is no single, internationally accepted system of classification for the lipids available. The names of these compounds, however, do fall into certain categories as the component structures present are considered. Bloor's classification is generally adopted with a few modifications as follows:

SIMPLE LIPIDS:
Simple lipids are esters of fatty acids with various alcohols. They contain mainly fatty acids and alcohols alone. They are further divided into two classes namely, Neutral fats and waxes.
Neutral fats:
They are triesters of fatty acids with glycerol. Triacyglycerol is an example for Neutral fats.
waxes:
Waxes are esters of fatty acids with higher mono hydroxy aliphatic alcohols. True waxes, cholesterol esters and vitamin A and D esters are example for waxes.
COMPOUND LIPIDS:
They are the esters of fatty acids containing groups, other than and in addition, to an alcohol and fatty acids.
a) PHOSPHOLIPIDS:
In addition to fatty acids and alcohol presence, they also contain phosphorous, nitrogenous bases and other substitution groups. Lecithin and cephalins are examples for phospholipids.
b) GLYCOLIPIDS:
Lipids containing carbohydrates are referred as glycolipids. They contain an special alcohol moiety called sphingosine or sphingol and nitrogenous base. They do not have phosphorous. Gangliosides and cerebrosides are examples of compounds lipids.
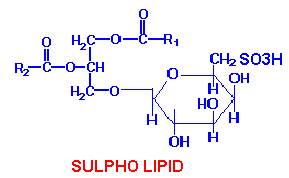
c) SULPHOLIPIDS:
Lipids with sulfate groups are referred as sulpholipids.
d) LIPOPROTEINS:
When lipids contain protein then they are known as lipoproteins. Example chylomicrons, VLDL, LDL and HDL.
DERIVED LIPIDS:
Derived lipids are lipids obtained upon hydrolysis of the simple and complex lipids and still retaining the characteristics of lipids. They are classified further into two types namely fattyacid and alcohol.
a) FATTY ACID:
They are the hydrolyzed products of simple and complex lipids. They mainly of mono carboxylic acids. They may be saturated or unsaturated. Their length varies between C4 to C30. Palmitic Acid C16
b) ALCOHOL:
It includes molecules with OH group as functional group. It also varies from simple straight chain alcohol like glycerol to complex cyclic alcohols like cholesterol.
MISCELLANEOUS:
This includes the lipids which can not be grouped under any of the above headings. They include
* Aliphatic hydrocarbons include iso-octadecane found in liver fat and certain hydrocarbons found in bees wax and plant waxes.
* Terpenes
* Carotenoids.
* Squalene is a hydrocarbon found in shark and mammalian liver and human sebum.
* Vitamin E and K.
SIMPLE LIPIDS:
FATS AND OILS:
Fats are the trihydric esters of the alcohol glycerol. They are also called as tri acyl glycerol or triglycerides. All the three OH groups of glycerol can be esterified with fatty acids.
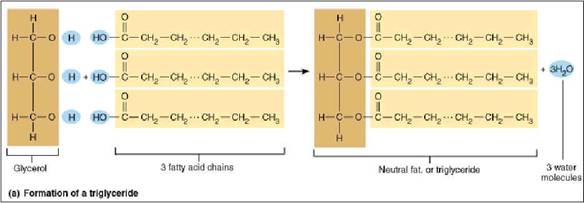
3 fatty acids + 1 glycerol = triglyceride
Depending upon the fatty acid residues in glycerol, there are two types of fats namely simple triglycerides and mixed triglycerides.
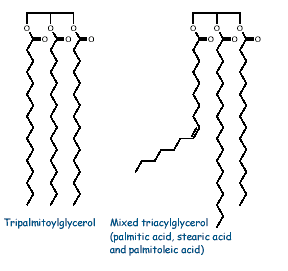
a) Simple triglycerides:
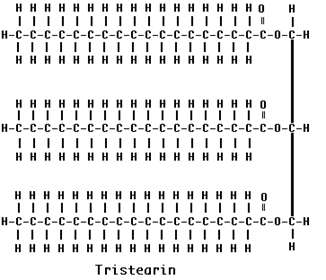
If all three fatty acids are the same, the triglyceride is called simple triglycerides. E.g. in Olive oil, the fatty acid is oleic acid then it was named as triolein. Similarly tristearin contains three stearic acid residues.
b) mixed triglycerides: If more than one fatty acid was esterified with glycerol then the fat is named as mixed triglycerides. The name of the fat depends upon the substituted fatty acid and its position. Mixed triglyerides exhibit isomerism depending on the position occupied by the different fatty acid residues.
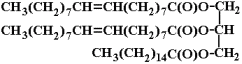
Artificial fats and fat substitutes have become more common as manufacturers target people who through misinformation have acquired aversions to fats or who would like to diet without reducing food intake. Olestra is an artificial fat created from sucrose (a carbohydrate) and up to eight fatty acids. In the olestra chemical structure, sucrose takes the place of glycerol. The olestra molecule is too large to be metabolized and passes through the body unchanged, but because it acts as a lipid, it can cause depletion of fat-soluble vitamins. Polyglycerol fatty acid esters (glyceran fatty acid esters) are mixtures of the esters of fatty acids with polyglycerol. These compounds have the general structure R-(OCH2-CH (OR)-CH2O)n-R, where R represents fatty acids and the average value of n is about 3. Polyglycerol fatty acid esters are almost completely metabolized like fats, so they are not calorie-free. The polymerized glycerol moiety is not digested and is excreted primarily in the urine. The main purpose of these compounds is to create products that are technically "fat free" and whose calories and fatty acid compositions are not reported on the Nutrition Facts of food labels. Generally vegetable oils are more unsaturated than animal fats (e.g. compare beef fat with corn and olive oils) but there are exceptions. I.e. sardine (animal) oil is highly unsaturated and coconut oil is highly saturated. These 'exceptions" can be rationalized by (i) sardines live in a cold environment and thus require more unsaturated fatty acid residues to stay fluid in vivo and (ii) coconut trees live in a hot environment and require more saturated fattyacid residues to stay fluid in vivo.
|
S. No |
ANIMAL FAT |
PLANT FAT |
|
1 |
Relatively rich in saturated fatty acids |
Relatively rich in unsaturated fatty acids. |
|
2 |
Solid at ordinary room temperature |
Liquid at room temperature |
|
3 |
These have usually low iodine number |
These have usually high iodine number |
|
4 |
These have usually high Reichert-Meissl Number |
These have low RM number |
|
5 |
These are stored mainly in liver and bone marrow |
These are stored mainly in seeds and fruits |
|
6 |
Oxidative rancidity is observed more frequently |
Oxidative rancidity is observed less frequently |
|
7 |
Example : Butter fat, Beef fat |
Example: Olive oil, Caster oil |
PROPERTIES OF FATS:
Properties of fats and oils depend upon the fatty acids and alcohol which are present in it. Properties of fats and oils are studied under two headings namely Physical and Chemical properties.
PHYSICAL PROPERTIES:
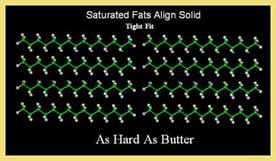
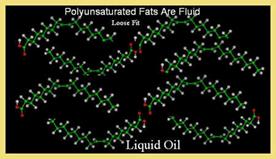
1. State:
Fats containing saturated fatty acids are solid at room temperature. The animal fats usually solid due to saturated fatty acids. Most plant fats, in contrast, possess unsaturated fatty acids and are, henceforth, liquid at room temperature.2. Color, odour and taste:
When pure, the fats are colorless, virtually odorless and possess an extremely bland taste. They are capable of absorbing a variety of odors and hence flavor during storage. For example, the perfumes of some flowers can be isolated by placing their petals in contact with the fat for a certain period, then extracting the fat with alcohol and concentrating the essence.
3. Solubility:
The fats are only sparingly soluble in water. These are, therefore, described as hydrophobic in contrast to the water soluble substances like proteins. However, these are freely soluble in organic solvents like chloroform, ether, acetone and benzene. These solvents, as they dissolve fats in them, are also known as fat solvents. The solubility of the fatty acids in organic solvents decreases with the increase of chain length. The introduction of hydroxyl groups, however, increases solubility.
4. Melting Point:
The melting point of fats depends on the chain length and the degree of unsaturation. Melting point increases with increase in their chain length but increase in the degree of unsaturation lowers melting point.
5. Specific gravity:
The specific gravity of the fats is less than 1 i.e. 0.86. Therefore, they float on water surface. Solid fats are lighter than the liquid fats.
6. Geometric Isomerism:
Presence of double bonds in unsaturated fatty acids is responsible for the geometric or cis-trans isomerism.
7. Insulation:
The fats possess high insulating power, i.e., they are bad conductor of heat. A layer of fat below the skin provides a sort of blanket for warm-blooded animals or homoiotherms. This is especially important for whales and seals which have to maintain a high temperature in cold wastes. The fishes are cold-blooded animals or poikiltherms and therefore, do not require maintenance of high temperature and so have very little subcutaneous fat.
8. Emulsification:
It is the process by which a lipid mass is converted into a number of small lipid droplets. The fats may be emulsified by shaking either with water or with emulsifying agents like soaps, gums, proteins, etc. An emulsifying agent helps in the production of a finely divided suspension of a fat in an aqueous medium. The hydrocarbon portions of the two tend to aggregate. This leaves the water-soluble group of the emulsifier projecting into the aqueous phase. A fat droplet will associate with a number of molecules of the emulsifier, thus producing a new water-soluble surface. Water molecules, henceforth, tend to be held in a layer or cloud around each droplet, thus disallowing the aggregation of the fat droplets. The process of emulsification is of great metabolic significance. In fact, the fats have to be emulsified before they can be absorbed by the intestinal wall. The process is accomplished by the bile juice secreted from liver.
9. Surface tension:
When liquid fat is poured on water, it spread uniformly over the surface of water in the form of a unimolecule layer and thus reduces the surface tension of water.
CHEMICAL PROPERTIES:
Chemical properties of fats studied under three different headings namely
1. Reactions due to COOH group
2. Reactions due to Double bonds
3. Reactions due to OH group
1. REACTIONS DUE TO –COOH GROUP:
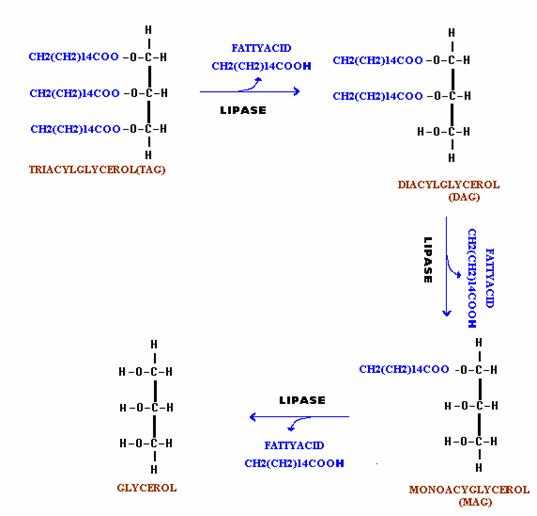
A. Hydrolysis by Enzyme:
The fats are hydrolyzed by the enzymes lipases to yield fatty acids and glycerol. This reaction occurs in three steps at a slightly alkaline pH. The fats first split to produce diglycerides, part of these are then split to monoglycerides. Finally, part of the monoglycerides split to yield to glycerol and fatty acid. This reaction plays significant role in digestion of lipids. In the intestine, the absorption of mono, di and tri glycerides is rapid, so that very little free glycerol is formed during fats digestion.
B. Hydrolysis by Alkali (Saponification):
The hydrolysis of fats by alkali is called as saponification. This reaction results in the formation of glycerol and salts of fatty acids which are called as soaps. The soaps are of two types namely hard and soft. Hard soaps such as the common bar soaps are the sodium salts of the higher fatty acids. Soft soaps are the potassium salts of higher fatty acids and are marketed as semisolids or pastes. The fatty acid salts of calcium, magnesium, zinc and lead are, however, insoluble in water. Calcium soaps are used industrially as lubricating greases. Zinc soaps are employed in the manufacture of talcum powder and other cosmetics. Lead and magnesium soaps are used in paints industry to hasten the process drying.
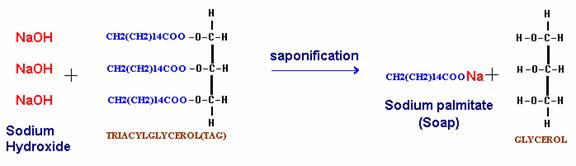
Soaps are important cleansing agents. Their cleansing property is due to their emulsifying action i.e. capacity to render more prolonged the mixing of oil and water. This is accomplished by means of negative charge the soap anion confers on oil droplets. The electrostatic repulsion then prevents the coalescence of soap and oil droplets into an oil phase.
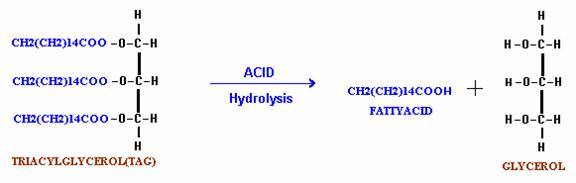
C. Hydrolysis by Acids:
When fats are hydrolyzed by acid, it yields only fatty acids and glycerol.
D. Hydrolysis by Microbes:
When butter or other fats are stored, they often become rancid and hence unpalatable. Rancidity is caused by the growth of microorganisms which secrete enzymes like lipases. These split the fats into glycerol and free fatty acids. The fatty acids impart unpleasant odour and flavor to the fat. Since, rancidity aroused due to the hydrolysis reaction, it is known as hydrolytic rancidity. However, butter may be prevented from becoming rancid by refrigeration or by exclusion of water.
2. REACTIONS DUE TO DOUBLE BONDS:
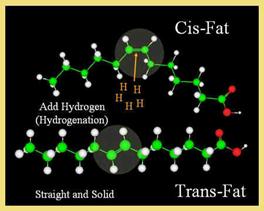
A. HYDROGENATION OF FATS:
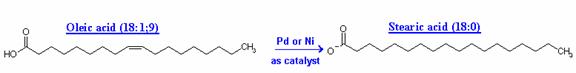
Unsaturated fats exposed to air oxidize to create compounds that have rancid, stale, or unpleasant odors or flavors. Hydrogenation is a commercial chemical process to add more hydrogen to natural unsaturated fats to decrease the number of double bonds and retard or eliminate the potential for rancidity. Unsaturated oils, such as soybean oil, which contain unsaturated fatty acids like oleic and linoleic acid, are heated with metal catalysts in the presence of pressurized hydrogen gas. Hydrogen is incorporated into the fatty acid molecules and they become saturated with hydrogen. Oleic acid (C18:1) and linoleic acid (C18:2) are both converted to stearic acid (C18:0) when fully saturated. The liquid vegetable oil becomes a solid saturated fat (shortening with a large percentage of tristearin). By comparison, animal fats seldom have more than 70% saturated fatty acid radicals.
![]()
Hydrogenation Process
Fully saturated fats are too waxy and solid to use as food additives, so manufacturers use partially hydrogenated oils. These oils are also produced at high temperatures with metal catalysts and pressurized hydrogen, but the process is stopped when the oil has the proper consistency for its application. The high temperatures and catalysts used for this chemical reaction weaken the double bonds and, as a side effect, cause a large percentage of the natural Cis double bonds to change to Trans double bonds. Trans fatty acids are present mainly in partially hydrogenated fats, but they are also present in hydrogenated fats because chemical reactions never achieve 100% efficiency.
B. Halogenation:
Unsaturated fatty acids and their esters can take up halogens like Bromine and Iodine at their double bonds at room temperature in acetic acid or methanol solution. This reaction is utilized to evaluate the unsaturation nature of fats in term of Iodine number.
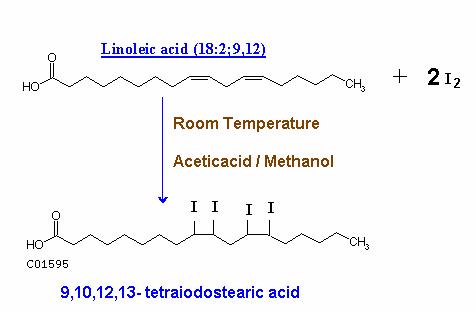
C. Oxidation:
Unsaturated fatty acids are susceptible to oxidation at their double bonds. Oxidation may be carried with ozone, KMnO4 and oxygen itself.
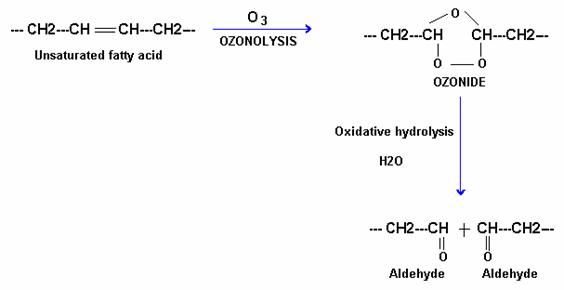
i) With ozone:
Ozone interacts with unsaturated fatty acids and form ozonide as an unstable intermediate and which is later cleaved by water to give two molecules of aldehydes.
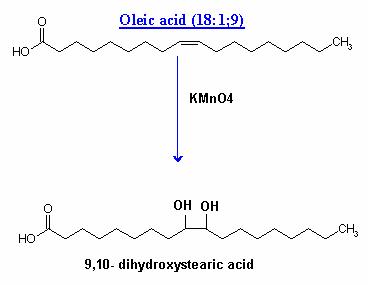
ii) With KMnO4 :
Under mild conditions, the glycols are formed at the sites of double bonds.
Under vigorous conditions, the same reagent cleaves the molecule at the double bond and oxidizes the terminal portions to the carboxyl group.
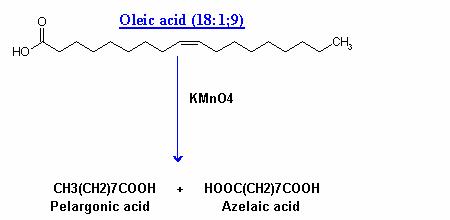
Oidation reactions utilized to find out the position of double bonds in fatty acid chain; this is useful in structural studies.
iii) With oxygen:
Oils containing highly unsaturated fatty acids are spontaneously oxidized by atmospheric oxygen at ordinary temperature. The oxidation takes place slowly and results in the formation of short chain fatty acids and aldehydes which give a rancid taste and odour to the fats. This type of rancidity or rancidification is called oxidative rancidity and is due to a reaction called autoxidation. Autoxidation proceeds by a free radical mechanism in which the alpha methylene group is primarily attacked. A hydrogen atom is removed from an alpha methylene group. This initiates a chain of reactions leading to oxidation.
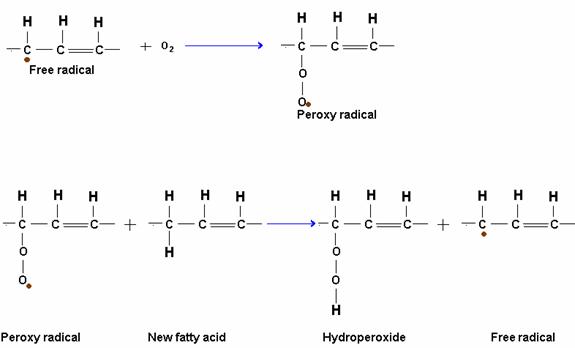
RANCIDITY:
The unpleasant odour and taste developed by most natural fats on aging is referred to as rancidity.
Causes of rancidity:
Rancidity may be caused by the following: firstly hydrolysis of fat yields free fatty acids and glycerol and / or mono and diglycerides. Process is enhanced by presence of lipolytic enzymes lipases, which in the presence of moisture and warm temperature bring about hydrolysis rapidly. Secondly by various oxidative processes, oxidation of double bonds of unsaturated glycerides may form “peroxides”, which then decompose to form aldehydes of objectionable odour and taste. The process is greatly enhanced by exposure to light.
Prevention of Rancidity:
Vegetable fats contain certain substances like vitamin E, phenols, hydroquinones, tannins and others which are antioxidants and prevent development of rancidity. Hence vegetable fats preserve for longer periods than animal fats.The action of antioxidants is opposed by a group of compounds present in the fats and oils. These accelerate the oxidation of the oxidation of the parent compound and are called as pro-oxidants. Majority of these substances are formed during the processing and refining of fats. Among the noteworthy pro-oxidants are the copper, iron and nickel salts of organic acids like lactic acid etc.
3. REACTIONS DUE TO HYDROXYL GROUP:
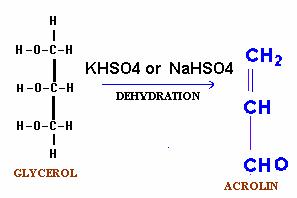
A. Dehydration (Acrolin test):
Fats when heated in the presence of a dehydrating agent, NaHSO4 or KHSO4 produce unsaturated aldehydes called acrolin from the glycerol moiety. Acrolin easily recognized by its pungent odour and thus forms the basis of the test for the presence of glycerol in fat molecule.
QUANTITATIVE TESTS:
The chemical reactions give valuable information about the chemical nature of fatty acids and the number of hydroxyl groups present in the fat molecule. Such chemical determinations involve various analytical tests. These are called chemical constants. They are used to identify a pure fat, assess the degree of adulteration and determine the proportions of different types of fat in a mixture. The constants used for these tests are include Saponification Number, Acid Number. Polenske Number, Reichert-Meissl Number, Iodine number and Acetyl Number.
1. Saponification Number:
The number of milligrams of KOH required to saponify the free and combined fatty acids in one gram of a given fat is called as its saponification number. The amount of alkali needed to saponify a given quantity of fat will depend upon the number of carboxylic group present. Thus fats containing short chain fatty acids will have more carboxylic groups per gram than long chain fatty acids and this will take up more alkali and hence will have higher saponification number. Butter containing a larger proportion of short chain fatty acids, such as butyric and caproic acids, has relatively high saponification number from 220 to 230. Oleo-margarine, with more long chain fatty acids, has saponification number of 195 or less.
2. Acid Number:
Number of milligrams of KOH required to neutralize the fatty acids in one gram of fat is known as the acid number. The acid number indicates the degree of rancidity of a given fat. The acid number, thus, determine the quantity of free fatty acid present in a fat. The fat which has been both processed and stored properly has a very low acid number.
3. Polenske Number:
The number of millilitre of 0.1N KOH required to neutralize the insoluble fatty acids which are those not volatile with steam distillation, present in 5 gram of fat is referred as Polenske number.
4. Reicher-Meissl Number:
It is the number of milliliters of 0.1N KOH required to neutralize the soluble volatile fatty acids which are distilled from 5 gram of fat. The Reichert-Meissl measures the amount of volatile soluble fatty acids. By saponification of fat, acidification and steam distillation, the volatile soluble acids may be separated and determined quantitatively. Butter fat is the only common fat with a high RM number and this determination, therefore, is of interest in that it aids the food chemist in detecting butter substitutes in food products.
5. Iodine Number:
Iodine Number is defined as the number of grams of iodine absorbed by 100 gram of fat. Iodine number is a measure of the degree of unsaturation of a fat. The more the iodine number, the greater the degree of unsaturation. The determination of iodine number is useful to the chemist in determining the quality of oil or its freedom from adulteration. Iodine number of cotton seed oil varies from 103 to 111 that of olive oil from 79 to 88, and that of linseed oil from 175 to 202. A commercial lot of olive oil which has iodine number higher than 88 might have been adulterated with cotton seed oil. Again a batch of linseed oil with iodine number lower than 175 might also have been adulterated with the cotton seed oil.
6. Acetyl Number:
The number of milligrams of KOH required to neutralize the acetic acid released by saponification of one gram of fat after it has been acetylated is known as acetyl number. Some of the fatty acid residues in fats contain hydroxyl groups. In order to determine the proportion of these, they are acetylated by means of acetic anhydride. Thus an acetyl group is introduced wherever a free –OH group is present. After washing out the excess acetic anhydride and acetic acid liberated, the acetylated fat can be dried and weighed and the acetic acid in combination determined by titration with standard alkali after it has been set free. The acetyl number is thus a measure of the number of hydroxyl group present.
Castor oil because of its high content of ricinoleic acid has a high acetyl number. Caster oil 146-150 and Olive oil 10.5. Acetyl number can be used to detect adulteration.
Synthesis of Triglycerides
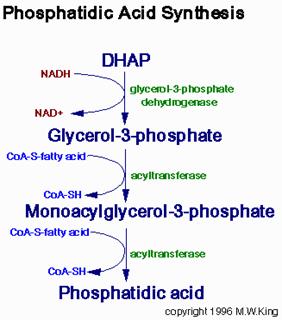
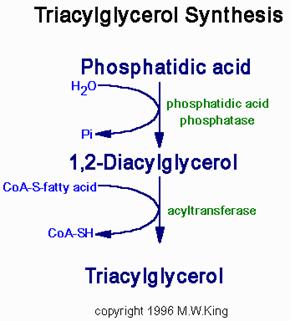
Fatty acids are stored for future use as triacylglycerols in all cells, but primarily in adipocytes of adipose tissue. Triacylglycerols constitute molecules of glycerol to which three fatty acids have been esterified. The fatty acids present in triacylglycerols are predominantly saturated. The major building block for the synthesis of triacylglycerols, in tissues other than adipose tissue, is glycerol. Adipocytes lack glycerol kinase, therefore, dihydroxyacetone phosphate (DHAP), produced during glycolysis, is the precursor for triacylglycerol synthesis in adipose tissue. This means that adipocytes must have glucose to oxidize in order to store fatty acids in the form of triacylglycerols. DHAP can also serve as a backbone precursor for triacylglycerol synthesis in tissues other than adipose, but does so to a much lesser extent than glycerol. The glycerol backbone of triacylglycerols is activated by phosphorylation at the C-3 position by glycerol kinase. The utilization of DHAP for the backbone is carried out through the action of glycerol-3-phosphate dehydrogenase, a reaction that requires NADH. The fatty acids incorporated into triacylglycerols are activated to acyl-CoAs through the action of acyl-CoA synthetases. Two molecules of acyl-CoA are esterified to glycerol-3-phosphate to yield 1,2-diacylglycerol phosphate (phosphatidic acid). The phosphate is then removed, by phosphatidic acid phosphatase, to yield 1, 2-diacylglycerol, and the substrate for addition of the third fatty acid. Intestinal monoacylglycerols, derived from the hydrolysis of dietary fats, can also serve as substrates for the synthesis of 1,2-diacylglycerols.
WAXES:
Waxes are esters of long chain fatty alcohols (reduced fatty acids) and fatty acids. E.g. carnauba wax and lanolin
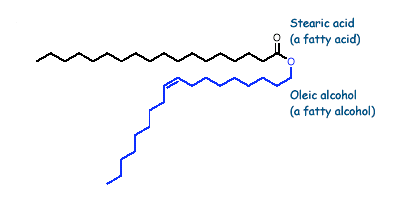
The word "wax" originate from the Old English word "Weax" meaning " the material of the honeycomb" i.e. beewax. The fatty acids rang in between C14 and C36 and the alcohols range from C16 to C36. Waxes are saponified with great difficulty than fats and are not attacked by lipase. Although waxes may be saponified by prolonged boiling with alcoholic KOH, they are more easily saponified by treating a solution of the wax in petroleum ether with absolute alcohol and metallic sodium i.e. with sodium ethoxide. The saponification products of waxes are water soluble soaps while the water insoluble long chain alcohols appear in the "unsaponifiable matter" fraction. Waxes contain about 31 - 55% of the unsaponifiable matter while fats and oils contain only 1-2% unsaponifiable matter. Bee wax: It contains esters derived from alcohols having 24 - 30 carbon atoms example palmitate of myricyl alcohol and n-hexacosanol
CH3 (CH2)14COOC30H61 MYRICYL PALMITATE
CH3 (CH2)14COOC26H53 N-HEXACOSANYL PALMITATE
Spermaceti: It is obtained from the head of the sperm whale. It is rich in ester of cetyl alcohol and palmitic acid. It is used in the manufacture of candle.
CH3 (CH2)14COOC16H33 CETYL PALMITATE
Sperm oil: It is a liquid wax and occurs with spermaceti in the sperm whale. It is a valuable lubricant used for delicate instruments such as watches. It does not become gummy like other normal oils.
Carnauba Wax: It is the hardest known wax. It consists mainly of fatty acids esterified with tetracosanol and tetratriacontanol. It is found in the leaves of the carnauba palm of Brazil. It is used as an ingredient in the manufacture of various wax polishes. Because waxes are very inert chemically, they make an excellent protective coating.
Lanolin: It is obtained from wool and is used in making ointments and salves. It readily forms an emulsion with water and for this reason makes it possible for drugs which are soluble in water to be incorporated into salves.
The waxes from the conifers contain polymers formed by the ester linking of many omega hydroxy acids such as Juniperic acid with each other.
HOOC (CH2)14CH2OH
JUNIPERIC ACID
There are certain waxes which have a characteristic odour. This is due to the presence of hydroxy acids in the form of lactones in them. For example a wax named ambretolide, which is extracted from the seeds of lady's finger, Ambelmoschus esculentus, has a characteristic musky smell. Lipids of marine organisms such as starfish, squid and shark contain fatty alcohols that are long chain alkyl ethers of glycerol. Three such glyceryl ethers, as they are called have been isolated from shark oil. Chinese wax is the secretion of an insect. Some of the waxes found in skin are esters of hydroxylated fatty acids and open chain alcohols. A wax found in blood plasma is found to have cholesteryl palmitate i.e. ester of cholesterol and palmitic acid.
CH3
(CH2)14CO
PHYSIOLOGICAL IMPORTANCE OF WAXES:
1. The most important physiological function of waxes is as a protective agent on the surfaces of animals and plants.
2. Waxes are found on the surface of feathers and hair with the result they remain soft and pliable.
3. Waxes prevent aquatic animals from becoming wet. Because of this water related birds like duck able to maintain dryness.
4. Waxy coating on the surface of plants protects them from excessive loss of moisture. Hence desert plants like palm and cactus can live for long periods with rain.
5. Waxy coating prevents the infection of plants from fungi and bacteria which can cause diseases.
6. waxy coating on several fruits like apples and citrus fruits prevent them from drying out and thus these fruits can be stored for long period of time. The waxy covering also protects such fruits from organism which cause rot.
COMPLEX LIPIDS:
PHOSPHOLIPIDS:
Phospholipids are of two types namely glycophospholipids and spingolipids depending upon the nature of the alcohol. If glycerol present as alcohol then they will be called as glycerophospholipids or glycophospholipids similarly if spingosine present as alcohol then they are known as spingophospholipids or spingolipids.
GLYCEROPHOSPHOLIPIDS:
Phospholipids or phosphatides are natural surfactants and emulsifiers consisting of an alcohol such as glycerol, one or two molecules of fatty acid, and a phosphoric acid compound. Phospholipids are fat derivatives in which one fatty acid has been replaced by a phosphate group and one of several nitrogen-containing molecules. They are found in all plants and animals and include such substances as lecithin, cephalin, and sphingomyelin. Lecithin is a significant constituent of brain and nervous tissue consisting of a mixture of diglycerides of stearic, palmitic, and oleic acids, linked to the choline ester of phosphoric acid. The chemical structure of dipalmitoyl lecithin illustrated here is typical of the phosphatides found in the brain, lung, and spleen.
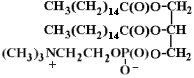
Lecithin
Example: Phosphatidyl ethanolamine (also known as cephalin)
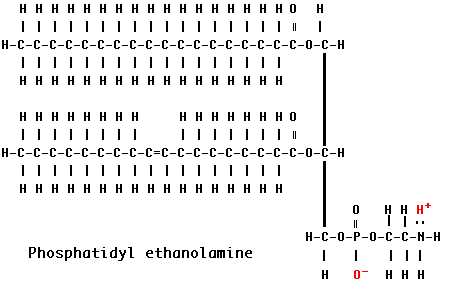
The hydrocarbon chains are hydrophobic . However, the charges on the phosphate and amino groups make that portion of the molecule hydrophilic. The result is an amphiphilic molecule. Amphiphilic lipids, in general, and phosphatidyl ethanolamine, in particular, are major constituents of cell membranes. These molecules form a phospholipid bilayer with their hydrophilic (polar) heads facing their aqueous surroundings (e.g., the cytosol) and their hydrophobic tails facing each other.
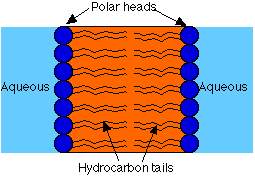
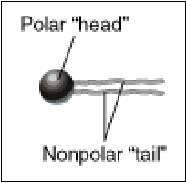
Properties of Lipid Bilayers
Permeability properties of bilayers are due in part to the asymmetry of the outer and inner layers. Compounds that dissolve more readily in oil have higher permeability Phase transition.Phospholipid Structures
Phospholipids are synthesized by esterification of an alcohol to the phosphate of phosphatidic acid (1, 2-diacylglycerol 3-phosphate). Most phospholipids have a saturated fatty acid on C-1 and an unsaturated fatty acid on C-2 of the glycerol backbone. The most commonly added alcohols (serine, ethanolamine and choline) also contain nitrogen that may be positively charged, whereas, glycerol and inositol do not. The major classifications of phospholipids are:
|
|
Phosphatidylcholine (PC) |
|
|
Phosphatidylethanolamine (PE) |
|
|
Phosphatidylserine (PS) |
|
|
Phosphatidylinositol (PI) |
|
|
Phosphatidylglycerol (PG) |
|
|
Diphosphatidylglycerol (DPG) |
Phospholipid Synthesis
Phospholipids can be synthesized by two mechanisms. One utilizes a CDP-activated polar head group for attachment to the phosphate of phosphatidic acid. The other utilizes CDP-activated 1, 2-diacylglycerol and an inactivated polar head group.
PC: This class of phospholipids is also called the lecithins. At physiological pH, phosphatidylcholines are neutral zwitterions. They contain primarily palmitic or stearic acid at carbon 1 and primarily oleic, linoleic or linolenic acid at carbon 2. The lecithin dipalmitoyllecithin is a component of lung or pulmonary surfactant. It contains palmitate at both carbon 1 and 2 of glycerol and is the major (80%) phospholipid found in the extracellular lipid layer lining the pulmonary alveoli. Choline is activated first by phosphorylation and then by coupling to CDP prior to attachment to phosphatidic acid. PC is also synthesized by the addition of choline to CDP-activated 1, 2-diacylglycerol. A third pathway to PC synthesis, involves the conversion of either PS or PE to PC. The conversion of PS to PC first requires decarboxylation of PS to yield PE; this then undergoes a series of three methylation reactions utilizing S-adenosylmethionine (SAM) as methyl group donor.
PE: These molecules are neutral zwitterions at physiological pH. They contain primarily palmitic or stearic acid on carbon 1 and a long chain unsaturated fatty acid (e.g. 18:2, 20:4 and 22:6) on carbon 2.Synthesis of PE can occur by two pathways. The first requires that ethanolamine be activated by phosphorylation and then by coupling to CDP. The ethanolamine is then transferred from CDP-ethanolamine to phosphatidic acid to yield PE. The second involves the decarboxylation of PS.
PS: Phosphatidylserines will carry a net charge of -1 at physiological pH and are composed of fatty acids similar to the phosphatidylethanolamines. The pathway for PS synthesis involves an exchange reaction of serine for ethanolamine in PE. This exchange occurs when PE is in the lipid bilayer of the membrane. As indicated above, PS can serve as a source of PE through a decarboxylation reaction.
PI: These molecules contain almost exclusively stearic acid at carbon 1 and arachidonic acid at carbon 2. Phosphatidylinositols composed exclusively of non-phosphorylated inositol exhibit a net charge of -1 at physiological pH. These molecules exist in membranes with various levels of phosphate esterified to the hydroxyls of the inositol. Molecules with phosphorylated inositol are termed polyphosphoinositides. The polyphosphoinositides are important intracellular transducers of signals emanating from the plasma membrane. The synthesis of PI involves CDP-activated 1,2-diacylglycerol condensation with myo-inositol. PI subsequently undergoes a series of phosphorylations of the hydroxyls of inositol leading to the production of polyphosphoinositides. One polyphosphoinositide (phosphatidylinositol 4, 5-bisphosphate, PIP2) is a critically important membrane phospholipid involved in the transmission of signals for cell growth and differentiation from outside the cell to inside.
PG: Phosphatidylglycerols exhibit a net charge of -1 at physiological pH. These molecules are found in high concentration in mitochondrial membranes and as components of pulmonary surfactant. Phosphatidylglycerol also is a precursor for the synthesis of cardiolipin. PG is synthesized from CDP-diacylglycerol and glycerol-3-phosphate. The vital role of PG is to serve as the precursor for the synthesis of diphosphatidylglycerols (DPGs).
DPG: These molecules are very acidic, exhibiting a net charge of -2 at physiological pH. They are found primarily in the inner mitochondrial membrane and also as components of pulmonary surfactant. One important class of diphosphatidylglycerols is the cardiolipins. These molecules are synthesized by the condensation of CDP-diacylglycerol with PG.The fatty acid distribution at the C-1 and C-2 positions of glycerol within phospholipids is continually in flux, owing to phospholipid degradation and the continuous phospholipid remodeling that occurs while these molecules are in membranes. Phospholipid degradation results from the action of phospholipases. There are various phospholipases that exhibit substrate specificities for different positions in phospholipids. In many cases the acyl group which was initially transferred to glycerol, by the action of the acyl transferases, is not the same acyl group present in the phospholipid when it resides within a membrane. The remodeling of acyl groups in phospholipids is the result of the action of phospholipase A1 and phospholipase A2. Cobra and bee venoms contain Phospholipase A2. These venoms, when injected into the blood, produce lysophospholipids that disrupt cellular membranes and lyse blood cells.
|
|
|
Sites of action of the phospholipases A1, A2, C and D. |
The products of these phospholipases are called lysophospholipids and can be substrates for acyl transferases utilizing different acyl-CoA groups. Lysophospholipids can also accept acyl groups from other phospholipids in an exchange reaction catalyzed by lysolecithin: lecithin acyltransferase (LLAT). Phospholipase A2 is also an important enzyme, whose activity is responsible for the release of arachidonic acid from the C-2 position of membrane phospholipids. The released arachidonate is then a substrate for the synthesis of the prostaglandins and leukotrienes.
Plasmalogens
Plasmalogens are glycerol ether phospholipids. They are of two types, alkyl ether and alkenyl ether. Dihydroxyacetone phosphate serves as the glycerol precursor for the synthesis of glycerol ether phospholipids. Three major classes of plasmalogens have been identified: choline, ethanolamine and serine plasmalogens. Ethanolamine plasmalogen is prevalent in myelin. Choline plasmalogen is abundant in cardiac tissue. One particular choline plasmalogen (1-alkyl, 2-acetyl phosphatidylcholine) has been identified as an extremely powerful biological mediator, capable of inducing cellular responses at concentrations as low as 10-11 M. This molecule is called platelet activating factor, PAF.
|
|
|
Platelet activating factor |
PAF functions as a mediator of hypersensitivity, acute inflammatory reactions and anaphylactic shock. PAF is synthesized in response to the formation of antigen-IgE complexes on the surfaces of basophils, neutrophils, eosinophils, macrophages and monocytes. The synthesis and release of PAF from cells leads to platelet aggregation and the release of serotonin from platelets. PAF also produces responses in liver, heart, smooth muscle, and uterine and lung tissues.
PHOSPHOLIPIDS IN DIGESTION:
Phospholipase A2 is secreted by the pancreas into the intestine. It hydrolyzes the ester linkage between the fatty acid and the hydroxyl on carbon 2 of phospholipids. Lysophospholipids, the products of Phospholipase A2 reactions, are powerful detergents. Lysophospholipids aid digestion of other lipids, by breaking up fat globules into small micelles. Some phospholipid (lecithin) is secreted by the liver in the bile, presumably to provide a substrate for Phospholipase A2 within the intestine, and thus aid in fat digestion.
EMULSIFYING AGENT:
An emulsifying agent is a substance which is soluble in both oil and water, thus enabling the two to mix. A “famous” phospholipid is lecithin which is found in egg yolk and soybeans. Egg yolk is mostly water but has a lot of lipids, especially cholesterol, which are needed by the developing chick. Lecithin is used to emulsify the lipids and hold them in the water as an emulsion. Lecithin is the basis of the classic emulsion known as mayonnaise.
ROLE OF PHOSPHOLIPIDS IN MEMBRANE
Phospholipids and cholesterol
are the principal components of nearly all cell membranes. The backbone of a
phospholipid is the same glycerol molecule that forms the backbone of
triglycerides. But instead of 3 fatty acids attached to glycerol, a phospholipid
consists of 2 fatty acids, a phosphate group and an alcohol. The most common
alcohols are derived from serine, ethanolamine, choline and inositol. Thus, the
most common phospholipids are phosphatidylcholine, phospatidylethanolamine,
phosphatidylserine, and phosphatidylinositol. Phosphatidylcholine is also known
as lecithin. "Commercial lecithin" (which is used as an emulsifying agent in
food processing) is a mixture of phospholipids from eggs, soybeans, nuts, etc.,
with phosphatidylcholine as the major ingredient.
Different cells have different
quantities of phospholipid in their membranes. Gray matter in the brain is
nearly 70% phospholipid, whereas brain white matter is less than half
phospholipid because of high concentrations of glycolipid (sugar-fat). There is
much variation in the amounts and kinds of phospholipid in membranes. Brain gray
matter is 30% phosphatidylcholine whereas brain white matter is 10%
phosphatidylcholine. The inner layer of neuron membranes primarily contain
phosphatidylethanolamine & phosphatidylserine, whereas in the outer layer
phosphatidylcholine & sphingomyelin predominate. Mitochondrial and endoplasmic
reticulum membranes are both 40% phosphatidylcholine. But mitochondrial
membranes are also 35% phosphatidylethanolamine, whereas endoplasmic reticulum
membrane is about 17% phosphatidylethanolamine.
Cells also vary considerably in the kinds of fatty acids attached to phosphatidic acid. In gray matter cell membranes, the fatty acids in the middle position of phospholipids are composed of carbon chains that are longer and more unsaturated than fatty acids found in the membrane phospholipids of most other cells. Fatty acids that are long and highly unsaturated increase membrane fluidity and functionality, which is why DHA and arachidonic acid are highly concentrated in the phospholipids of neuron synapses. Unsaturated fatty acids are also important for membrane activity at the site of hormone receptors. Insulin resistance in adult-onset diabetes is associated with fewer membrane long-chain unsaturated fatty acids due to impaired desaturase and elongase enzyme function.
|
Phospholipids in Cell Membranes |
|
|
|
|
The alcohol portion of a phospholipid protrudes away from the membrane, whereas the two fatty acids jut into the membrane. The middle fatty acid (in the second position) is usually unsaturated (like arachidonic acid) whereas the end fatty acid (in the first position) is usually saturated (like stearic acid).
ACTION OF PHOSPHOLIPASES:
Each of the 3 groups attached to the glycerol
backbone has a special enzyme that can separate the group from the backbone.
Phospholipase A1 enzyme attacks the attachment of the first fatty
acid, Phospholipase A2 attacks the attachment of the middle
fatty acid and Phospholipase D attacks the alcohol attachment.
Phospholipase C -- which is a major toxin secreted by bacteria -- releases
1, 2-DiAcylGlycerol (DAG) along with a phosphoryl base.
|
Phospholipase Enzymes in Cell Membranes |
|
|
|
|
Frequently the unsaturated fatty acid stored in the second position of a cell membrane will be arachidonic acid, EPA or DHA (especially in the neurons). The release of arachidonic acid or EPA from cell membranes by Phospholipase A2 allows the enzymes lipoxygenase and cyclooxygenase to form biologically active eicosanoids like prostaglandins (PGZs, first isolated in prostate gland), thromboxanes (TXZs, first isolated in thrombocytes) and leucotrienes (LTZs, first isolated in leucocytes). These eicosanoids can be compared to hormones, except that unlike hormones they are destroyed by local enzymes within seconds or minutes after formation. This limits the activities of eicosanoids to the area where they were released. Having cell membranes contain fatty acids that can form the hormone-like eicosanoids gives the body the capacity to produce quick, localized action in almost any tissue or organ. The most general need for rapid, local action is the response to trauma. Therefore, eicosanoids are most often concerned with clotting, inflammation and the initiation of immune defense.
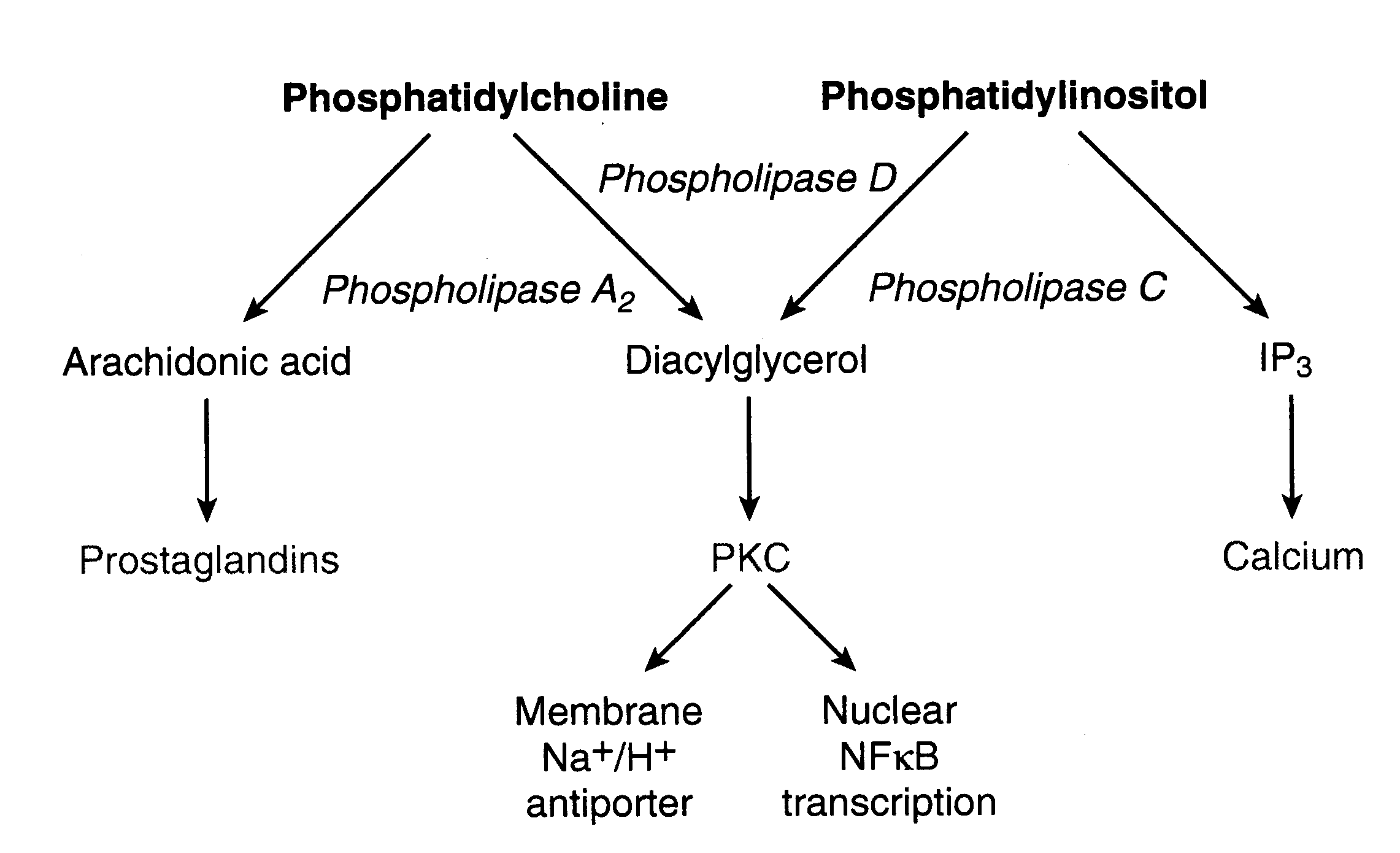
Some membrane phospholipids, such as the phosphatidylinositols, function to convert activity at cell surface G-protein-coupled receptors into intracellular signals. Hydrolysis by phospholipase C and phospholipase D produce the second messengers (intracellular messengers) DiAcylGlycerol (DAG) [which stimulates Protein Kinase C (PKC)] and Inositol triPhosphate (IP3) [which causes intracellular release of calcium]. Activated PKC concentrates in the plasma membrane where it phosphorylates membrane proteins of receptors and ion-channels to inhibit their function (negative feedback). Nuclear Factor kB (NFkB) activated by PKC binds to DNA promoters & enhancers of inflammatory cytokines, among other genes. IP3 binds to the endoplasmic reticulum, releasing calcium stored in that location. DAG can be further hydrolyzed by Phospholipase A2 to release more arachidonic acid. Ethanol increases Phospholipase A2 activity, increasing oxidative stress.
Three categories of PhosphoLipase A2 (PLA2) are recognized: secretory PLA2 (sPLA2), cytoplasmic PLA2 (cPLA2) and Ca2+-independent PLA2 (iPLA2). The cPLA2 is activated by Ca2+ to a much greater extent than sPLA2. Whereas sPLA2 is more prominent in inflammatory disease, cPLA2 is more associated with oxidative free radical damage. The cPLA2 shows a marked preference for hydrolyzing oxidized arachidonic acid in cell membranes, which may be important for membrane maintenance when sufficient ATP is available to synthesize and insert fresh arachidonic acid into membranes. Arachidonic acid is a particularly common constituent of brain neuron membranes and the massive release of arachidonic acid in cerebral cortex ischemia/reperfusion plays a significant role in exacerbating ischemic/reperfusion damage. The activity of cPLA2 on inner mitochondrial membranes can also be exacerbated under conditions of high oxidative stress.
FUNCTIONS OF PHOSPHOLIPIDS:
1. Structural Role:
Phospholipids participate in the lipoprotein complexes which are thought to constitute the matrix of cell walls and membranes, the myelin sheath, and of such structures as mitochondria and microsomes. In this role, they impart certain physical characteristics like high permeability towards certain nonpolar molecules and Lysis by surface active agents like detergents, bile salts etc.
2. Role in enzyme action:
Certain enzymes require tightly bound phospholipids for their actions, e.g. mitochondrial enzyme system involved in oxidative phosphorylation.
3. Role in blood coagulation:
Phospholipids play an important part in the blood coagulation process. They are required at the stage of conversion of prothrombin by active factor X and in the activation of factor VIII by activated factor IX. Platelets provide the chief source of phospholipids and that part of total lipid content of the platelets which contribute to intrinsic blood coagulation process is called platelet factor 3.
4. Role in lipid absorption in intestine:
Lecithin lowers the surface tension of water and aids in emulsification of lipid water mixture, a prerequisite in digestion and absorption of lipids from gastrointestinal tract.
5. Role in transport of lipids from intestines:
Exogenous triglycerides is carried as lipoprotein complex, chylomicrons, in which phospholipids takes an active part.
6. Role in transport of lipids from liver:
Endogenous triglycerides are carried from Liver to various tissues as lipoprotein complex pre-beta-lipoprotein. Phospholipid is required for the formation of the lipoprotein complex.
7. Role in electron transport:
Probably phospholipid helps to couple oxidation with phosphorylation and maintains electron transport enzymes in active conformation and proper relative positions.
8. Lipotrophic action of Lecithin:
Choline acts as a lipotropic agent as it can prevent formation of fatty liver. As lecithin can provide choline it acts as a lipotropic agent.
9. Ion transport and secretion:
Phospholipids are in some way implicated in the mechanism of secretion is suggested by the observation that phospholipids, specially phosphatidic acid and phosphor-inositides turnover is proportional to the rate of secretion of cells liberating such products as hormones, enzymes, mucins and other proteins.
10. Membrane phospholipids:
Phospholipids of membrane are hydrolyzed by phospholipase A2 and provide the unsaturated fatty acid arachidonic acid, which is utilized for synthesis of prostaglandins and leukotrienes.
11. Insulation:
Phospholipids of myelin sheaths provide the insulation around the nerve fibers.
12. Cofactor:
Phospholipids are required as a cofactor for the activity of the enzyme lipoprotein lipase and triacylglycerol lipase.
12. Role in Hormone action:
Some signal must provide communication between the hormone receptor on the plasma membrane and intracellular Calcium reservoirs. The best candidate appears to be the products of phosphatidyl inositides metabolism. Phosphatidyl inositol-4, 5-bisphosphate is hydrolyzed to myo-inositol1, 4, 5-triphosphate and diacylglycerol through the action of a phosphodiesterase. Myo-inositol-triphosphate releases calcium ions from a variety of membrane and organelle preparations.
SPINGOPHOSPHOLIPIDS:
The sphingolipids, like the phospholipids, are composed of a polar head group and two nonpolar tails. The core of sphingolipids is the long-chain amino alcohol, sphingosine. Amino acylation, with a long chain fatty acid, at carbon 2 of sphingosine yields a ceramide.
|
Sphingosine |
|
Ceramide |
The sphingolipids include the sphingomyelins and glycosphingolipids (the cerebrosides, sulfatides, globosides and gangliosides). Sphingomyelins are the only sphingolipid that are phospholipids. Sphingolipids are a component of all membranes but are particularly abundant in the myelin sheath. Sphingomyelins are sphingolipids that are also phospholipids. Sphingomyelins are important structural lipid components of nerve cell membranes. The predominant sphingomyelins contain palmitic or stearic acid N-acylated at carbon 2 of sphingosine.The sphingomyelins are synthesized by the transfer of phosphorylcholine from phosphatidylcholine to a ceramide in a reaction catalyzed by sphingomyelin synthase.
|
|
|
A sphingomyelin |
Defects in the enzyme acid sphingomyelinase result in the lysosomal storage disease known as Niemann-Pick disease. There are at least 4 related disorders identified as Niemann-Pick disease Type A and B (both of which result from defects in acid sphingomyelinase), Type C1 and a related C2 and Type D. Types C1, C2 and D do not result from defects in acid sphingomyelinase.
GLYCOLIPIDS:
They are type of complex lipids where phosphate found to be absent and moreover alcohol is spingosine. Glycosphingolipids, or glycolipids, are composed of a ceramide backbone with a wide variety of carbohydrate groups (mono- or oligosaccharides) attached to carbon 1 of sphingosine. The four principal classes of glycosphingolipids are the cerebrosides, sulfatides, globosides and gangliosides.
CERBROSIDES (Glycospingosides):
Cerebrosides have a single sugar group linked to ceramide. The most common of these is galactose (galactocerebrosides), with a minor level of glucose (glucocerebrosides). Galactocerebrosides are found predominantly in neuronal cell membranes. By contrast glucocerebrosides are not normally found in membranes, especially neuronal membranes; instead, they represent intermediates in the synthesis or degradation of more complex glycosphingolipids.
There is no glycerol, no phosphoric acid and no nitrogenous base. Thus on hydrolysis, cerebroside yields a sugar, usually galactose but sometimes glucose, a high molecular weight fatty acid and the alcohol, spingosine or dihydrospingosine. Thus they contain nitrogens though there is no nitrogenous base i.e in the form of spingosine. Individual cerebrosides are differentiated on the basis of their fatty acid component. There are four different types namely Kerasin, Phreosin , Nervon and Oxynervon. Kerasin contains saturated fatty acid i.e Lignoceric acid with 24 carbon atoms. Phrenosin (Cerebron) contains a hydroxyl fatty acid i.e cerbronic acid with 24 carbon atoms. Cerebronic acid is nothing but 2 hydroxy lignoceric acid. Nervon contains an unsaturated fattyacid namely nervonic acid. It is homologue to lignoceric acid but it contains double bond at C 15. Oxynervon contains hydroxyl fatty acid oxynervonic acid which is derived from nervonic acid by hydroxylation at C-2.
By prolonged hydrolysis of any cerebroside with Ba(OH)2, fatty acid is removed and it yields psychosin which is nothing but complex of spingosine and sugar. Psychosine can be further hydrolyzed to yield spingosine and galactose. Galactocerebrosides are synthesized from ceramide and UDP-galactose. Excess accumulation of glucocerebrosides is observed in Gaucher's disease.
|
|
|
A Galactocerebroside |
Sulfatides: The sulfuric acid esters of galactocerebrosides are the sulfatides. Sulfatides are synthesized from galactocerebrosides and activated sulfate, 3'-phosphoadenosine 5'-phosphosulfate (PAPS). Excess accumulation of sulfatides is observed in sulfatide lipidosis (metachromatic leukodystrophy).
Globosides: Globosides represent cerebrosides that contain additional carbohydrates, predominantly galactose, glucose or GalNAc. Lactosyl ceramide is a globoside found in erythrocyte plasma membranes. Globotriaosylceramide (also called ceramide trihexoside) contains glucose and two moles of galactose and accumulates, primarily in the kidneys, of patients suffering from Fabry's disease.
Gangliosides: Kelnk, in 1942, isolated from beef brain, a new class of carbohydrate rich glycolipids which he called as gangliosides. Gangliosides have been isolated from fanglion cells, neuronal bodies and dendrites, spleen and RBC stroma. The highest concentrations are found in gray matter of brain. Gangliosides are the most complex of the glycospingolipids. They are large complex lipids. Their molecular weights varies from 180,000 to 250,000. Although the exact structure of the gangliosides are not definitely established. On hydrolysis, gangliosides yield the following:
Brain gangliosides are known to be complex and mono, di, trisialogangliosides containing 1 to 3 sialic acid residues have been isolated. Gangliosides are very similar to globosides except that they also contain NANA in varying amounts. The specific names for gangliosides are a key to their structure. The letter G refers to ganglioside, and the subscripts M, D, T and Q indicate that the molecule contains mono-, di-, tri and quatra (tetra)-sialic acid. The numerical subscripts 1, 2 and 3 refer to the carbohydrate sequence that is attached to ceramide; 3 stands for GalGalNAcGalGlc-ceramide, 2 for GalNAcGalGlc-ceramide and 1 for GalGlc-ceramide.
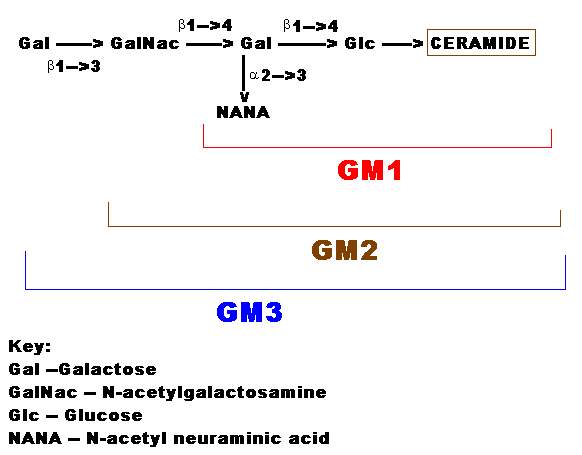
The simplest and common ganglioside found in tissues is GM1, which contains ceramide, one molecule of glucose, one molecule of galactose and one molecule of NANA. GM3 is a more complex ganglioside derived from GM1 is of considerable biologic interest, as it is now known to be the receptor in human intestine for cholera toxin. Gangliosides are mainly components of, membranes. The sugar units and sialic acid sections of the molecule are water soluble and negatively charged, whereas the ceramide portion is lipid soluble. The lipid soluble part appears to be embedded in the membrane lipids whereas other component with its charged units protrudes externally towards the medium. The gangliosides, therefore, can serve as specific membrane binding sites for circulating hormones and thereby influence various biochemical processes in the cell.
Deficiencies in lysosomal enzymes, which normally are responsible for the degradation of the carbohydrate portions of various gangliosides, underlie the symptoms observed in rare autosomally inherited diseases termed lipid storage diseases, many of which are listed below.
SULPHO LIPIDS:
A glycolipid that contains sulfur is widely distributed in plants. It is localized in the chloroplast but is also found in the chromatophores of photosynthetic bacteria. As the sulfur in this compound is present as a sulfonic group in a hexose, this may be included under a class of compounds called sulfolipids.
Clinical Significances of Sphingolipids and Phospholipids:
One of the most clinically important classes of sphingolipids is those that confer antigenic determinants on the surfaces of cells, particularly the erythrocytes. The ABO blood group antigens are the carbohydrate moieties of glycolipids on the surface of cells as well as the carbohydrate portion of serum glycoproteins. When present on the surface of cells the ABO carbohydrates are linked to sphingolipid and are therefore of the glycosphingolipid class. When the ABO carbohydrates are associated with protein in the form of glycoproteins they are found in the serum and are referred to as the secreted forms. Some individuals produce the glycoprotein forms of the ABO antigens while others do not. This property distinguishes secretors from non-secretors, a property that has forensic importance such as in cases of rape. A significant cause of death in premature infants and, on occasion, in full term infants is respiratory distress syndrome (RDS) or hyaline membrane disease. This condition is caused by an insufficient amount of pulmonary surfactant. Under normal conditions the surfactant is synthesized by type II endothelial cells and is secreted into the alveolar spaces to prevent atelectasis following expiration during breathing. Surfactant is comprised primarily of dipalmitoyllecithin; additional lipid components include phosphatidylglycerol and phosphatidylinositol along with proteins of 18 and 36 kDa (termed surfactant proteins). During the third trimester the fetal lung synthesizes primarily sphingomyelin, and type II endothelial cells convert the majority of their stored glycogen to fatty acids and then to dipalmitoyllecithin
|
|
|
Structure of the ABO blood group carbohydrates R represents the
linkage to protein in the secreted forms |
Fetal lung maturity can be determined by measuring the ratio of lecithin to sphingomyelin (L/S ratio) in the amniotic fluid. An L/S ratio less than 2.0 indicates a potential risk of RDS. The risk is nearly 75-80% when the L/S ratio is 1.5. The carbohydrate portion of the ganglioside, GM1, present on the surface of intestinal epithelial cells, is the site of attachment of cholera toxin, the protein secreted by Vibrio cholerae. These are just a few examples of how sphingolipids and glycosphingolipids are involved in various recognition functions at the surface of cells. As with the complex glycoproteins, an understanding of all of the functions of the glycolipids is far from complete.
Disorders Associated with Abnormal Sphingolipid Metabolism
|
Disorder |
Enzyme Deficiency |
Accumulating Substance |
Symptoms |
|
Tay-Sachs disease |
HEXA |
GM2 ganglioside |
rapidly progressing mental retardation, blindness, early mortality |
|
Sandhoff-Jatzkewitz disease |
HEXB |
Globoside, GM2 ganglioside |
same symptoms as Tay-Sachs, progresses more rapidly |
|
Tay-Sachs AB variant |
GM2 activator (GM2A) |
GM2 ganglioside |
same symptoms as Tay-Sachs |
|
Gaucher's disease |
Glucocerebrosidase |
Glucocerebroside |
hepatosplenomegaly, mental retardation in infantile form, long bone degeneration |
|
Fabry's disease |
a-Galactosidase A |
Globotriaosylceramide; also called ceramide trihexoside (CTH) |
kidney failure, skin rashes |
|
Niemann-Pick disease, more info below |
|
|
all types lead to mental retardation, hepatosplenomegaly, early fatality potential |
|
Krabbe's disease; globoid leukodystrophy |
Galactocerebrosidase |
Galactocerebroside |
mental retardation, myelin deficiency |
|
GM1 gangliosidosis |
GM1 ganglioside:b -galactosidase |
GM1 ganglioside |
mental retardation, skeletal abnormalities, hepatomegaly |
|
Sulfatide lipodosis; |
Arylsulfatase A |
Sulfatide |
mental retardation, metachromasia of nerves |
|
Fucosidosis |
a-L-Fucosidase |
Pentahexosylfucoglycolipid |
cerebral degeneration, thickened skin, muscle spasticity |
|
Farber's lipogranulomatosis |
Acid ceramidase |
Ceramide |
hepatosplenomegaly, painful swollen joints |
The GM2 gangliosidoses include Tay-Sachs disease, the Sandhoff diseases and the GM2 activator deficiencies. GM2 ganglioside degradation requires the enzyme b-hexosaminidase and the GM2 activator protein (GM2A). Hexosaminidase is a dimer composed of 2 subunits, either a and/or b. The HexS protein is a, HexA is ab and HexB is bb. It is the a-subunit that carries out the catalysis of GM2 gangliosides. The activator first binds to GM2 gangliosides followed by hexosaminidase and then digestion occurs. Based upon genetic linkage analyses as well as enzyme studies and the characterization of accumulating lysosomal substances, Niemann Pick disease should be divided into type I and type II; type I has 2 subtypes, A and B (NPA and NPB), which show deficiency of acid sphingomyelinase. Niemann Pick disease type II likewise has 2 subtypes, type C1 and C2 (NPC) and type D (NPD). It is obviously confusing to use the abbreviation NPD for Niemann Pick disease in some cases and for subtype D of Niemann Pick disease in other cases.
LIPOPROTEINS:
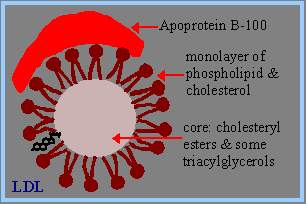
Free fatty acids are transported in the blood bound to albumin, a serum protein secreted by the liver. Most other lipids are transported in the blood as part of complex particles called lipoproteins. The general structure of a lipoprotein includes, a core consisting of a droplet of triacylglycerols and/or cholesteryl esters a surface monolayer of phospholipid, unesterified cholesterol and specific proteins (apolipoproteins, e.g., apoprotein B-100 in low density lipoprotein).Lipoproteins differ in their content of proteins and lipids. They are classified based on their density:
Chylomicrons
Chylomicrons are assembled in the intestinal mucosa as a means to transport dietary cholesterol and triacylglycerols to the rest of the body. Chylomicrons are, therefore, the molecules formed to mobilize dietary (exogenous) lipids. The predominant lipids of chylomicrons are triacylglycerols (see Table above). The apolipoproteins that predominate before the chylomicrons enter the circulation include apoB-48 and apoA-I, -A-II and IV. ApoB-48 combines only with chylomicrons. Chylomicrons leave the intestine via the lymphatic system and enter the circulation at the left subclavian vein. In the bloodstream, chylomicrons acquire apoC-II and apoE from plasma HDLs. In the capillaries of adipose tissue and muscle, the fatty acids of chylomicrons are removed from the triacylglycerols by the action of lipoprotein lipase (LPL), which is found on the surface of the endothelial cells of the capillaries. The apoC-II in the chylomicrons activates LPL in the presence of phospholipid. The free fatty acids are then absorbed by the tissues and the glycerol backbone of the triacylglycerols is returned, via the blood, to the liver and kidneys. Glycerol is converted to the glycolytic intermediate dihydroxyacetone phosphate (DHAP). During the removal of fatty acids, a substantial portion of phospholipid, apoA and apoC is transferred to HDLs. The loss of apoC-II prevents LPL from further degrading the chylomicron remnants. Chylomicron remnants--- containing primarily cholesterol, apoE and apoB-48--- are then delivered to, and taken up by, the liver through interaction with the chylomicron remnant receptor. The recognition of chylomicron remnants by the hepatic remnant receptor requires apoE. Chylomicrons function to deliver dietary triacylglycerols to adipose tissue and muscle and dietary cholesterol to the liver.
Very Low Density Lipoproteins, VLDLs
The dietary intake of both fat and carbohydrate, in excess of the needs of the body, leads to their conversion into triacylglycerols in the liver. These triacylglycerols are packaged into VLDLs and released into the circulation for delivery to the various tissues (primarily muscle and adipose tissue) for storage or production of energy through oxidation. VLDLs are, therefore, the molecules formed to transport endogenously derived triacylglycerols to extra-hepatic tissues. In addition to triacylglycerols, VLDLs contain some cholesterol and cholesteryl esters and the apoproteins, apoB-100, apoC-I, apoC-II, apoC-III and apoE. Like nascent chylomicrons, newly released VLDLs acquire apoCs and apoE from circulating HDLs. The fatty acid portion of VLDLs is released to adipose tissue and muscle in the same way as for chylomicrons, through the action of lipoprotein lipase. The action of lipoprotein lipase coupled to a loss of certain apoproteins (the apoCs) converts VLDLs to intermediate density lipoproteins (IDLs), also termed VLDL remnants. The apoCs are transferred to HDLs. The predominant remaining proteins are apoB-100 and apoE. Further loss of triacylglycerols converts IDLs to LDLs.
Intermediate Density Lipoproteins, IDLs
IDLs are formed as triacylglycerols are removed from VLDLs. The fate of IDLs is either conversion to LDLs or direct uptake by the liver. Conversion of IDLs to LDLs occurs as more triacylglycerols are removed. The liver takes up IDLs after they have interacted with the LDL receptor to form a complex, which is endocytosed by the cell. For LDL receptors in the liver to recognize IDLs requires the presence of both apoB-100 and apoE (the LDL receptor is also called the apoB-100/apoE receptor). The importance of apoE in cholesterol uptake by LDL receptors has been demonstrated in transgenic mice lacking functional apoE genes. These mice develop severe atherosclerotic lesions at 10 weeks of age.
Low Density Lipoproteins, LDLs
The cellular requirement for cholesterol as a membrane component is satisfied in one of two ways: either it is synthesized de novo within the cell, or it is supplied from extra-cellular sources, namely, chylomicrons and LDLs. As indicated above, the dietary cholesterol that goes into chylomicrons is supplied to the liver by the interaction of chylomicron remnants with the remnant receptor. In addition, cholesterol synthesized by the liver can be transported to extra-hepatic tissues if packaged in VLDLs. In the circulation VLDLs are converted to LDLs through the action of lipoprotein lipase. LDLs are the primary plasma carriers of cholesterol for delivery to all tissues. The exclusive apolipoprotein of LDLs is apoB-100. LDLs are taken up by cells via LDL receptor-mediated endocytosis, as described above for IDL uptake. The uptake of LDLs occurs predominantly in liver (75%), adrenals and adipose tissue. As with IDLs, the interaction of LDLs with LDL receptors requires the presence of apoB-100. The endocytosed membrane vesicles (endosomes) fuse with lysosomes, in which the apoproteins are degraded and the cholesterol esters are hydrolyzed to yield free cholesterol. The cholesterol is then incorporated into the plasma membranes as necessary. Excess intracellular cholesterol is re-esterified by acyl-CoA-cholesterol acyltransferase (ACAT), for intracellular storage. The activity of ACAT is enhanced by the presence of intracellular cholesterol.
Insulin and tri-iodothyronine (T3) increase the binding of LDLs to liver cells, whereas glucocorticoids (e.g., dexamethasone) have the opposite effect. The precise mechanism for these effects is unclear but may be mediated through the regulation of apoB degradation. The effects of insulin and T3 on hepatic LDL binding may explain the hypercholesterolemia and increased risk of atherosclerosis that have been shown to be associated with uncontrolled diabetes or hypothyroidism.
An abnormal form of LDL, identified as lipoprotein-X (Lp-X), predominates in the circulation of patients suffering from lecithin-cholesterol acyl transferase (LCAT, see HDL discussion for LCAT function) deficiency or cholestatic liver disease. In both cases there is an elevation in the level of circulating free cholesterol and phospholipids. As VLDL particles are transported through the bloodstream, Lipoprotein Lipase catalyzes triacylglycerol removal by hydrolysis. With removal of triacylglycerols as well as some proteins, the percentage of weight that is cholesteryl esters increases. VLDL is converted to IDL and eventually to LDL.
VLDL --> IDL --> LDL
High Density Lipoproteins, HDLs
HDLs are synthesized de novo in the liver and small intestine, as primarily protein-rich disc-shaped particles. These newly formed HDLs are nearly devoid of any cholesterol and cholesteryl esters. The primary apoproteins of HDLs are apoA-I, apoC-I, apoC-II and apoE. In fact, a major function of HDLs is to act as circulating stores of apoC-I, apoC-II and apoE. HDLs are converted into spherical lipoprotein particles through the accumulation of cholesteryl esters. This accumulation converts nascent HDLs to HDL2 and HDL3. Any free cholesterol present in chylomicron remnants and VLDL remnants (IDLs) can be esterified through the action of the HDL-associated enzyme, lecithin:cholesterol acyltransferase, LCAT. LCAT is synthesized in the liver and so named because it transfers a fatty acid from the C-2 position of lecithin to the C-3-OH of cholesterol, generating a cholesteryl ester and lysolecithin. The activity of LCAT requires interaction with apoA-I, which is found on the surface of HDLs. Cholesterol-rich HDLs return to the liver, where they are endocytosed. Hepatic uptake of HDLs, or reverse cholesterol transport, may be mediated through an HDL-specific apoA-I receptor or through lipid-lipid interactions. Macrophages also take up HDLs through apoA-I receptor interaction. HDLs can then acquire cholesterol and apoE from the macrophages; cholesterol-enriched HDLs are then secreted from the macrophages. The added apoE in these HDLs leads to an increase in their uptake and catabolism by the liver. HDLs also acquire cholesterol by extracting it from cell surface membranes. This process has the effect of lowering the level of intracellular cholesterol, since the cholesterol stored within cells as cholesteryl esters will be mobilized to replace the cholesterol removed from the plasma membrane. The cholesterol esters of HDLs can also be transferred to VLDLs and LDLs through the action of the HDL-associated enzyme, cholesterol ester transfer protein (CETP). This has the added effect of allowing the excess cellular cholesterol to be returned to the liver through the LDL-receptor pathway as well as the HDL-receptor pathway.
DERIVED LIPIDS:
FATTY ACIDS:
Fatty acids consist of the elements carbon (C), hydrogen (H) and oxygen (O) arranged as a carbon chain skeleton with a carboxyl group (-COOH) at one end. A fatty acid may be defined as an organic acid that occurs in a natural triglyceride and is a monocarboxylic acid ranging in chain length from C4 to about C30 carbon atoms. Fatty acids usually do not occur in free or uncombined state in cells or tissues but are present in covalently bound form in different classes of lipids. Fatty acids which occur in natural fats are usually monocarboxylic and contain an even number of carbon atoms since they are synthesized from 2 carbon precursor acetyl coA.
NOMENCLATURE:
The systematic nomenclature of the fatty acids is based on the Genevan system. According to this system, the fatty acid is named after the hydrocarbon with the same number of carbon atoms, the suffix “–oic” is written in place of the final letter “e” in the name of the hydrocarbon. The names of saturated fatty acids end with the suffix “-anoic” and those of unsaturated acids with the suffix “-enoic”. The position of carbon atoms in the fatty acid chain is indicated either by numbering in which case the carboxyl carbon is numbered as c1, the carbon adjacent to C1 as C2 and so on or by the use of Greek letters in which case carbon atom next to carboxyl group numbered as alpha and next carbon beta and so on. The methyl carbon of fatty acid labeled as omega carbon.
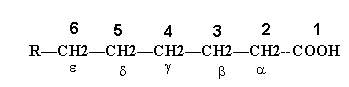
Generally, the numbers are used with the International Union of Pure and Applied Chemistry (IUPAC) system of nomenclature and the Greek letters when the common names are employed.
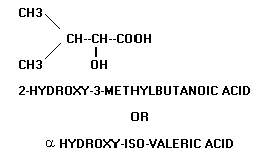
For example:
A widely used convention to indicate the number and position of the double bonds in the case of unsaturated fatty acids is to write number of carbon atoms, the number of double bonds and the position of the double bonds below the name of the acid. The position of a double bond is indicated by the lower number of the two carbon atoms involved in double bonding. For example, oleic acid having 18 carbon atoms and a double bond between carbon atoms 9 and 10 is written as 18:1;9. Similarly linoleic acid with 18 carbon atoms, 2 double bonds at C9 and C12 is written as 18:2;9,12. An alternative method to write the name of an unsaturated fatty acid is to write first the position of double bonds in numerals and then the total number of carbon atoms in Roman followed by the suffix “-enoic” acid. Thus, oleic acid may be written as 9-octadecenoic acid and Linoleic acid as 9,12-octadecadienoic acid.
Fatty acid classified as follows:
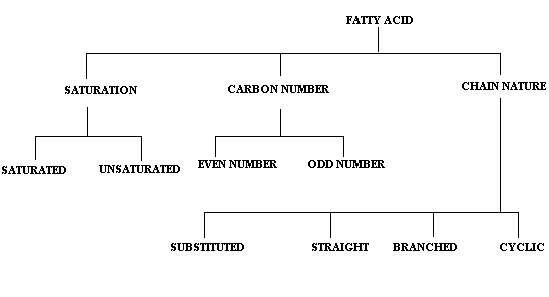
SATURATION:
On the basis of the state of saturation in fatty acids, they are studied under two different headings namely saturated and unsaturated fatty acids.
SATURATED FATTY ACIDS:
The general formula for these acids is CnH2n+1COOH. When there is no double or triple bonds are present in the fatty acids they are referred as saturated fatty acids. They vary from C3 to C30. Saturated fatty acids having 10 carbons or less number of carbon atoms are called as lower fatty acids e.g. acetic acid and butyric acid. Milk contains significant amount of lower fatty acids. Saturated fatty acids having more than 10 carbon atoms are called higher fatty acids e.g. palmitic acid and stearic acid
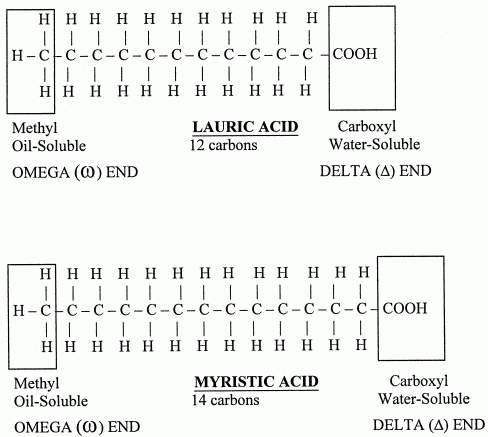
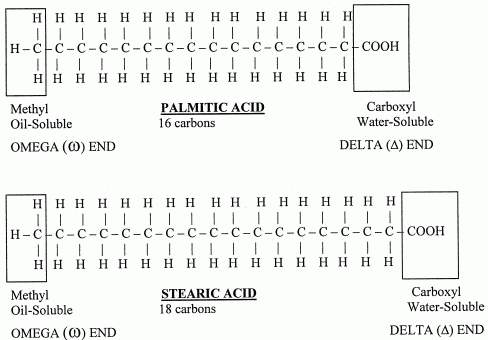
UNSATURATED FATTY ACIDS:
Fatty acids with only one double-bond are called mono-unsaturated. And fatty acids with more than one double-bond are called poly-unsaturated. Monounsaturated or monoethenoid acids contain single double bond in their structure. The general formula for monounsaturated fatty acids is CnH2n-1COOH. Examples: Palmitoleic acid and Oleic acid. Polyunsaturated fatty acids (PUFA) contain more than one double bonds in their structure. Depending upon the double bonds their general formulae vary. For example PUFA with two double bonds general formula is CnH2n-3COOH. Similarly for three double bond containing fatty acid is CnH2n-5COOH and for four double bonds CnH2n-7COOH.
A most unusual unsaturated fatty acid, nemotinic acid, is excreted in the growth medium by a citrivorium mold. This fatty acid is unique in that it contains the single, double and triple C—C linkages. Nemotinic acid is one of the few naturally occurring compounds containing the allene group.
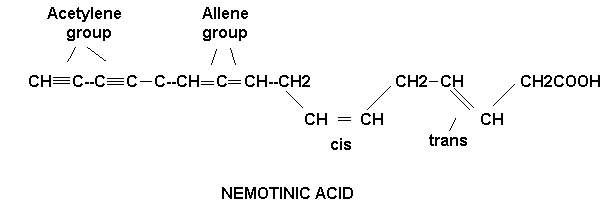
On account of the presence of double bonds, the unsaturated fatty acids exhibit geometric or cis-trans isomerism. Most unsaturated fatty acids are found as the unstable cis isomer rather than as the more stable than trans isomer.
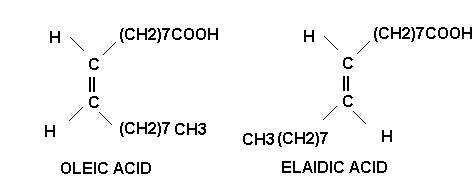
Another structural peculiarity of naturally occurring polyunsaturated fatty acids is the presence of a non conjugated double bond system. It has a methylene group (-CH2-) flanked by double bonds on both the sides as in linoleic, linolenic and arachidonic acids. The conjugated double bond system is, however, rarely present. In it the methylene group is not found in between the double bonds which, hence forth, occur one after the other as in eleostearic acid. This acid has valuable properties as a drying oil since it polymerizes readily. These two double bond systems have different chemical properties.
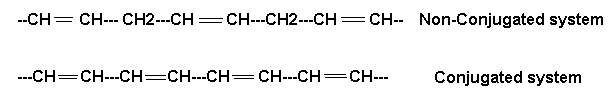
Polyunsaturated fats have often been recommended to reduce coronary heart disease [*9]. But all saturated fats do not have the same effect on cholesterol synthesis in the liver. Only the saturated fats of chain-length 12, 14 and 16 (lauric acid, myristic acid and palmitic acid) have been shown to elevate blood cholesterol. Of these, myristic acid (high in coconut and palm oil) elevates cholesterol the most [*10]. Stearic acid (18-carbon, saturated) has been shown to lower cholesterol by 21% -- even more than oleic acid (18-carbon, mono-unsaturated) which lowers LDL by 15% [*11].
Polyunsaturated fatty acids can be a health hazard because carbon-carbon double bonds can lead to free-radical formation and reactions with oxygen to form unstable lipid peroxide compounds containing the same unstable oxygen-oxygen bond found in hydrogen peroxide. Lipid peroxidation and free radicals can cause cancer and may accelerate aging. High rates of lung cancer among women in China have been associated with lipid peroxidized oils in fumes from cooking polyunsaturated vegetable oils in a wok [*12]. Hot oil in open air is subject to much lipid peroxidation. Fast-food restaurants that fry foods in the same oil all day serve lots of lipid peroxides to their customers.
Polyunsaturated "cis" fatty acids can be beneficial in cell membranes by preventing the tight packing of fatty acids in membranes -- thereby making the membranes more "fluid". Membrane fluidity is important for optimal function of most cells in the body. But membrane fluidity is especially important on portions of cells that act as receptors for hormones or neurotransmitters. The typical North American eats three times as much saturated fat as unsaturated fat, yet animal experiments show that insulin receptor responsiveness is substantially improved when dietary unsaturated fat is greater than saturated fat [*13]. With aging, however, cell membrane fluidity declines in part because of increasing amount of cholesterol in the membranes, but more importantly because of free-radical oxidation [*14]. Antioxidants that protect cell membranes, like Vitamin E, are extremely valuable in opposing membrane oxidation.
Fatty acid double-bonds come in two configurations known as cis (carbon chains on the same side of a double-bond) and trans (carbon chains on the opposite side of a double-bond). Most of the double-bonds made by biological systems have the cis configuration. It is the cis configuration of unsaturated fatty acids that prevents tight packing of fatty acids in membranes, and hence increases membrane fluidity. Saturated fats (like butter or lard) and fatty acids with trans double-bonds (like margarine) tend to be solids at room temperature, whereas natural fatty acids with cis double-bonds (like vegetable oils) tend to be liquids. By artificially hydrogenating vegetable oils, the food processing industry reduces the number of double bonds and causes the formation of trans fatty acids. Hydrogenation results in margarines that are more solid and results in peanut butter that does not have oil that separates. But when trans fatty acids are incorporated into cell membranes, the membrane fluidity is reduced and the cells do not function as well. Not all trans fatty acids in the diet are due to food processing. For example, natural butter is 5% trans fat.
NUMBER OF CARBON ATOMS:
Depending upon the number of carbon atoms, fatty acids generally classified into even number and odd number fatty acids.
EVEN CHAIN FATTY ACIDS:
Fatty acids with even number carbon atoms are referred as even number fatty acids. Examples are palmitic acid and stearic acid
ODD CHAIN FATTY ACIDS:
If the number of carbon atoms in fatty acid is odd number then the fatty acids are refered as odd chain fatty acids. Example: propionic acid and pentanoic acid
CHAIN NATURE:
Depending upon the nature of carbon chain in fatty acids, they are classified as straight, branched, cyclic and substituted fatty acids.
STRAIGHT CHAIN FATTY ACIDS:
If the carbon chain in fatty acids is straight chain then they are referred as straight chain fatty acids. Example palmitic acid and stearic acid
BRANCHED CHAIN FATTY ACIDS:
If the carbon chain in fatty acids is branched in nature then they are referred as branched chain fatty acids. Example Isopalmitic acid in wool fat and phytanic acid in butter. Phytanic acid is 3, 7, 11, 15 tetramethyl hexadecanoic acid.
SUBSTITUTED FATTY ACIDS:
In hydroxy fatty acid and methyl fatty acid, one or more of the hydrogen atoms have been replaced by -OH group or -CH3 group respectively. Both saturated and unsaturated hydroxy fatty acids, particularly with long chains, are found in nature e.g. cerebronic acid of brain glycolipids. Ricinoleic acid in castor oil.

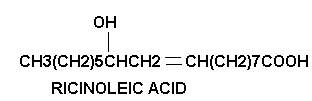
CYCLIC FATTY ACIDS:
These are rare occurrence. Chaulmoogra oil, obtained from the plant Hydnocarpus kurzil and used in the treatment of leprosy, contains two such acids hydnocarpic and chaulmoogric acids. They are named so because they contain cyclic structure.
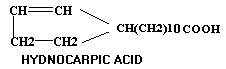
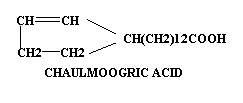
Lipids from the lactobacilli contain a fatty acid, lactobacillic acid, with a cyclopropyl group. This fatty acid may result from the addition of a methylene group across the double bond of vaccenic acid (18:1; 11)
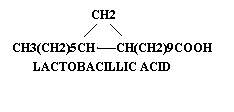
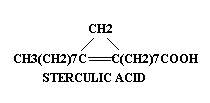
Similarly, Sterculic acid from plant sources has a comparable structure, with a suggested relationship to oleic acid. It may be derived from oleic acid by the addition of a methylene group across the double bond in a manner that the unsaturated nature is not altered, unlike the lactobacillic acid.
STRUCTURE OF FATTY ACIDS:
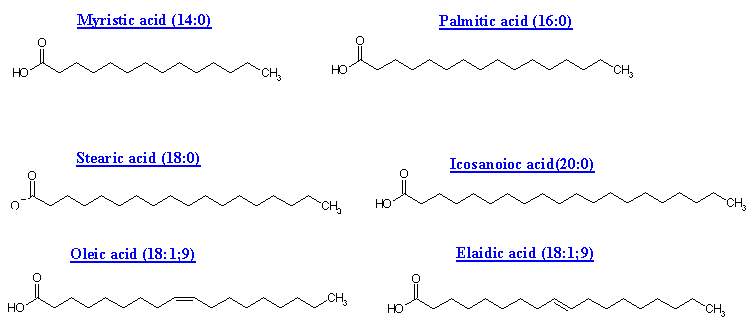
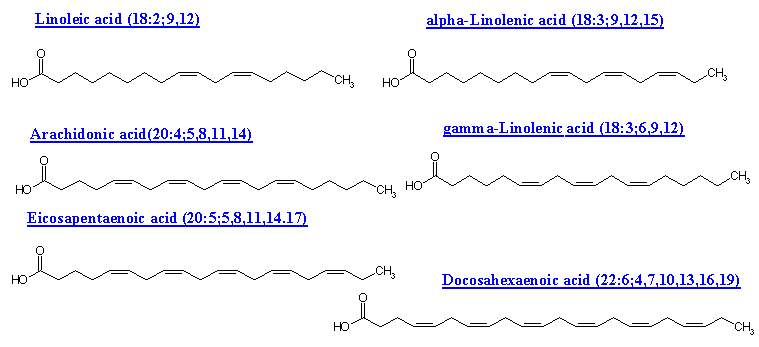
|
Chemical Names and Descriptions of some Common Fatty Acids |
||||
|
Common Name |
Carbon |
Double |
Scientific Name |
Sources |
|
Butyric acid |
4 |
0 |
butanoic acid |
butterfat |
|
Caproic Acid |
6 |
0 |
hexanoic acid |
butterfat |
|
Caprylic Acid |
8 |
0 |
octanoic acid |
coconut oil |
|
Capric Acid |
10 |
0 |
decanoic acid |
coconut oil |
|
Lauric Acid |
12 |
0 |
dodecanoic acid |
coconut oil |
|
Myristic Acid |
14 |
0 |
tetradecanoic acid |
palm kernel oil |
|
Palmitic Acid |
16 |
0 |
hexadecanoic acid |
palm oil |
|
Palmitoleic Acid |
16 |
1 |
9-hexadecenoic acid |
animal fats |
|
Stearic Acid |
18 |
0 |
octadecanoic acid |
animal fats |
|
Oleic Acid |
18 |
1 |
9-octadecenoic acid |
olive oil |
|
Vaccenic Acid |
18 |
1 |
11-octadecenoic acid |
butterfat |
|
Linoleic Acid |
18 |
2 |
9,12-octadecadienoic acid |
safflower oil |
|
Alpha-Linolenic Acid |
18 |
3 |
9,12,15-octadecatrienoic acid |
flaxseed (linseed) |
|
Gamma-Linolenic Acid |
18 |
3 |
6,9,12-octadecatrienoic acid |
borage oil |
|
Arachidic Acid |
20 |
0 |
eicosanoic acid |
peanut oil, |
|
Gadoleic Acid |
20 |
1 |
9-eicosenoic acid |
fish oil |
|
Arachidonic Acid (AA) |
20 |
4 |
5,8,11,14-eicosatetraenoic acid |
liver fats |
|
EPA |
20 |
5 |
5,8,11,14,17-eicosapentaenoic acid |
fish oil |
|
Behenic acid |
22 |
0 |
docosanoic acid |
rapeseed oil |
|
Erucic acid |
22 |
1 |
13-docosenoic acid |
rapeseed oil |
|
DHA |
22 |
6 |
4,7,10,13,16,19-docosahexaenoic |
fish oil |
|
Lignoceric acid |
24 |
0 |
tetracosanoic acid |
small amounts |
Butyric acid (butanoic acid) is one of the saturated short-chain fatty acids responsible for the characteristic flavor of butter. This image is a detailed structural formula explicitly showing four bonds for every carbon atom and can also be represented as the equivalent line formulas:
CH3CH2CH2COOH or CH3 (CH2)2COOH
The numbers at the beginning of the scientific names indicate the locations of the double bonds. By convention, the carbon of the carboxyl group is carbon number one. Greek numeric prefixes such as di, tri, tetra, penta, hexa, etc., are used as multipliers and to describe the length of carbon chains containing more than four atoms. Thus, "9, 12-octadecadienoic acid" indicates that there is an 18-carbon chain (octa deca) with two double bonds (di en) located at carbons 9 and 12, with carbon 1 constituting a carboxyl group (oic acid). The structural formula corresponds to:
CH3CH2CH2CH2CH2CH=CHCH2CH=CHCH2CH2CH2CH2CH2CH2CH2COOH
9, 12-octadecadienoic acid (Linoleic Acid)
Which would be abbreviated as:
CH3 (CH2)4CH=CHCH2CH=CH (CH2)7COOH
Fatty acids are frequently represented by a notation such as C18:2 that indicate that the fatty acid consists of an 18-carbon chain and 2 double bonds. Although this could refer to any of several possible fatty acid isomers with this chemical composition, it implies the naturally-occurring fatty acid with these characteristics, i.e., linoleic acid. When two double bonds are separated by one single bond they are said to be "conjugated". The term "conjugated linoleic acid" (CLA) refers to several C18:2 linoleic acid variants such as 9, 11-CLA and 10, 12-CLA which correspond to 9, 11-octadecadienoic acid and 10, 12-octadecadienoic acid.
Double bonds bind carbon atoms tightly and prevent rotation of the carbon atoms along the bond axis. This gives rise to configurational isomers which are arrangements of atoms that can only be changed by breaking the bonds.
|
|
|
|
Cis-9-octadecenoic acid |
Trans-9-octadecenoic acid |
|
|
|
These three-dimensional molecular projections show the Cis and Trans configurational isomers of 9-octadecenoic acid with the hydrogen atoms shown in blue. The Latin prefixes Cis and Trans describe the orientation of the hydrogen atoms with respect to the double bond. Cis means "on the same side" and Trans means "across" or "on the other side". Naturally occurring fatty acids generally have the Cis configuration. The natural form of 9-octadecenoic acid (oleic acid) found in olive oil has a "V" shape due to the Cis configuration at position 9. The Trans configuration (elaidic acid) looks more like a straight line.
|
|
|
|
Cis Configuration |
Trans Configuration |
Omega-3 (ω3) and omega-6 (ω6) fatty acids are unsaturated "Essential Fatty Acids" (EFAs) that need to be included in the diet because the human metabolism cannot create them from other fatty acids. Since these fatty acids are polyunsaturated, the terms n-3 PUFAs and n-6 PUFAs are applied to omega-3 and omega-6 fatty acids, respectively. These fatty acids use the Greek alphabet (α, β, γ, ω) to identify the location of the double bonds. The "alpha" carbon is the carbon closest to the carboxyl group (carbon number 2), and the "omega" is the last carbon of the chain because omega is the last letter of the Greek alphabet. Linoleic acid is an omega-6 fatty acid because it has a double bond six carbons away from the "omega" carbon. Similarly, alpha-linolenic acid is an omega-3 fatty acid because it has a double bond three carbons away from the "omega" carbon. By subtracting the highest double-bond location in the scientific name from the number of carbons in the fatty acid we can obtain its classification. For arachidonic acid, we subtract 14 from 20 to obtain 6; therefore, it is an omega-6 fatty acid. This type of terminology is sometimes applied to oleic acid which is an omega-9 fatty acid.
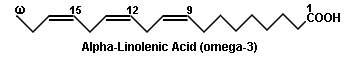
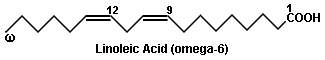
In these simplified structural formulas of unsaturated fatty acids, each angle represents a carbon atom. Notice that all the double bonds have the Cis configuration. DHA (docosahexaenoic acid) and AA (arachidonic acid) are both crucial to the optimal development of the brain and eyes. The importance of DHA and AA in infant nutrition is well established, and both substances are routinely added to infant formulas. Excessive amounts of omega-6 polyunsaturated fatty acids and a very high omega-6/omega-3 ratio have been linked with pathogenesis of many diseases, including cardiovascular disease, cancer, and inflammatory and autoimmune diseases. The ratio of omega-6 to omega-3 in modern diets is approximately 15:1, whereas ratios of 2:1 to 4:1 have been associated with reduced mortality from cardiovascular disease, suppressed inflammation in patients with rheumatoid arthritis, and decreased risk of breast cancer.
Function of Essential Fatty acids:
1. Poly unsaturated fatty acids occur in higher concentration in lipids associated with structural elements of tissues.
2. Lipids of gonads also contain high concentration of polyunsaturated fatty acids, which suggests importance of these compounds in reproductive function.
3. Prostaglandins are synthesized from Arachidonic acid by cyclooxygenase enzyme system. Leukotrienes are conjugated trienes formed from Arachidonic acid in leucocytes by the lipoxygenase pathway.
4. Essential fatty acids found to be the important component of the mitochondrial membrane. Because deficiency of these leads to the swelling of mitochondrial membrane and reduction in the activity of the mitochondrion especially energy production.
5. Fats with high content of poly unsaturated fattyacid tend to lower serum level of cholesterol.
6. Prolongation of clotting time is noted in ingestion of fats rich in essential fatty acids.
7. An increase in fibrinolytic activity follows the ingestion of fats rich in essential fatty acids.
8. Increased level of essential fatty acids prevents the occurrence of fatty liver.
9. Docosahexenoic acid formed from linolenic acid is the most abundant fatty acids present in retinal photoreceptor membranes. It enhances the electrical response of the photoreceptors to illumination.
DEFICIENCY MANIFESTATIONS:
A deficiency of EFA has not yet been demonstrated in humans. In weaning animals, symptoms of EFA deficiency are readily produced. They are: