TRANSPLANTATION
Transplantation refers to the act of transferring cells, tissues or organs from one site to another within individuals or between different individuals or between different species of genetically identical or not. The tissue or organ transplanted is known as the transplant or graft. The individual from whom the graft is obtained is known as the donor and the individual on whom it is applied known as recipient or acceptor. After transplantation, if the graft is survived in the recipient then the graft said to be accepted and it the graft is not survived then the graft is said to be rejected. Rejected graft usually in white color, so it is called as white graft.
There are mainly four different types of grafts namely Autograft, Isograft (Syngraft), Allograft (Homograft) and Xenograft (Heterograft).
Autograft: It is self tissue transferred from one body site to another in the same individual. These grafts are often performed on patients with burns by transferring healthy skin to the burned area. Always graft is accepted.
Isograft: It is the tissue transferred between genetically identical individuals. In humans, this type of graft transfer possible usually between identical (Monozygotic) twins. This type of graft was also accepted. This type of graft is formerly known as syngraft or syngenic graft.
Allograft: It is the tissue transferred between genetically non-identical members of the same species. Most of the organs (grafts) transplanted in human populations are found to be allograft. Normally allgorafts are rejected after transplantation, but immunosuppressive drugs and immune tolerance properties utilized for the survival of graft. This graft is also otherwise known as homograft by considering the factor that the graft is transferred within species.
Xenograft: It is the tissue transferred between different species. This graft is also normally rejected. Pigs’ hearth volve usually transplanted to humans. Through immunosuppressive and tolerance properties, graft survived in transplanted individuals. This graft is also otherwise called as heterograft due to the transfer of tissues or organs from one species to other species.
Based on the anatomical site of origin of the transplant and the site of its placement, grafts are classified as orthotopic and heterotopic. Orthotopic grafts are applied in anatomically normal sites. Ex: skin grafts. Heterotopic grafts are placed in anatomically abnormal sites, ex: when thyroid tissue is transplanted in a subcutaneous pocket. Transplants may be of living or dead materials. Live grafts, such kidney or heart, are expected to survive and function physiologically in the recipient and are called vital grafts. Nonliving transplants like bone or artery merely provide scaffolding on which new tissue is laid by the recipient. They are called static or structural grafts.
Transplantation Antigens:
Tissues that are antigenitically similar are said to be histocompatible such tissues do not induce an immunologic response that leads to graft rejection. Tissues displaying significant antigenic differences are histoincompatible; such tissues induce immune response leading to tissue rejection. Antigens that participate in graft rejection are called as transplantation or histocompatibility antigens. The blood group antigens are important in transplantation. The term major histocompatibility system is applied to a system of cell antigens that exert a decisive influence on the fate of allografts. Major histocompatibility systems have been identified in different species – H2 in mice, AgB in rats, B in chickens, H1 in rabbits and DLA in dogs. The major histocompatibility system in human beings is the human leucocytes antigen (HLA) system.
The various antigens that determine histocompatibility are encoded by more than 40 different loci, but the loci responsible for the most vigorous allograft rejection are located within the major histocompatibility complex (MHC). MHC identity of donor and host is not the sole factor determining tissue acceptance. When tissue is transplanted between genetically different individuals, even if their MHC antigens are identical, the transplanted tissue is likely to be rejected because of differences at various minor histocompatibility loci. There may be polymorphic antigens other than MHC molecules that differ between the donor and the recipient. These antigens induce weak or slower rejection reactions than do MHC molecules and are called minor histocompatibility antitens. Most minor histocompatibility antigens are proteins that are processed and presented to host T cell in association with self MHC molecules on host APCs.
GRAFT REJECTION:
Usually autografts and isografts are accepted and allograft and xenografts are rejected. Graft rejection can be studied in different headings like specificity and memory response to graft rejection, time variation in graft rejection and actual mechanism of graft rejection.
SPECIFICITY AND MEMORY OF REJECTION:
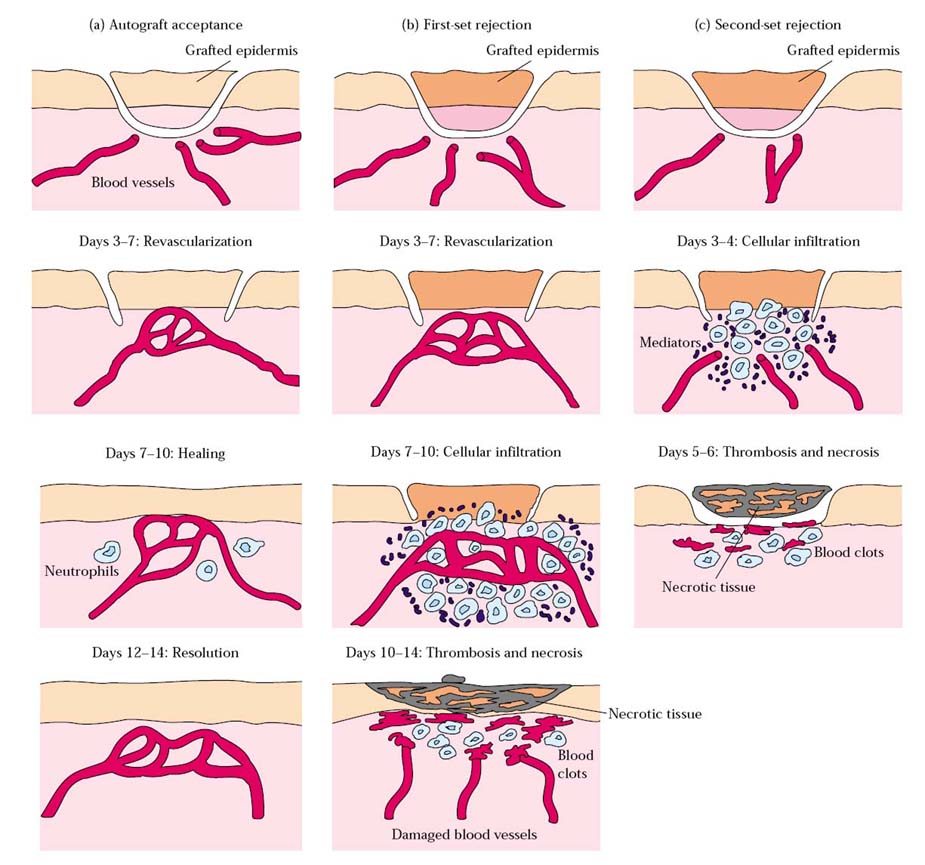
When a graft is transplanted first time, it is referred as primary graft. If the primary graft is allograft type then it is rejected within 14 days of transplantation. The rejection type is known as first set of rejection. When another graft from same donor is transplanted second time or more then the graft is said to be secondary graft and it is rejected with 5-6 days. The rejection type is known as second set of rejection. The variation in the rejection time periods is because of immunologic memory and specificity between grafts.
For example: If a strain-A inbred mouse is grafted with skin from strain B, primary graft rejection, known as first set rejection occurs. As the reaction develops, the vasculaeized transplant becomes infiltrated with lymphocytes, monocytes, and other inflammatory cells; there is decreased vascularization of the transplanted tissue by 6-9 days, visible necrosis by 10 days and complete rejection by 14 days. Immunologic memory is demonstrated when a second strain-B graft is transferred to a previously grafted strain-A mouse. In this case, a graft-rejection reaction develops more quickly than after the first graft, with complete rejection occurring with 5-6 days; this secondary response is designated second set rejection. The specificity of second set rejection can be demonstrated by grafting an unrelated strain C graft at the same time as the second strain-B graft. Rejection of the strain–C graft proceeds according to first set rejection kinetics, whereas stain-B graft is rejected in an accelerated second set fashion.

TIME COURSE IN GRAFT REJECTION:
Graft rejection reactions have various time courses depending upon the type of tissue or organ grafted and the immune response involved. Hyperacute rejection reaction occur within the first 24hr after transplantation; acute rejection reactions usually begin in the first few weeks after transplantation; and chronic rejection reaction can occur from months to years after transplantation.
Hyperacute Rejection:
Hyperacute Rejection is characterized by thrombotic occlusion of the graft vasculature that begins with minutes to hours after host blood vessels are anastomosed to graft vessels and is mediated by preexisting antibodies in the host circulation that bind to donor endothelial antigens. Binding of antibody to endothelium activates complement and antibody and complement induce a number of changes in the graft endothelium that promote intravascular thrombosis. Complement activation leads to endothelial cell injury and exposure of subendothelial basement membrane proteins that activate platelets. The endothelial cells are stimulated to secrete high molecular weight forms of von Willebrand factor that mediate platelets adhesion and aggregation. Both endothelial cells and platelets undergo membrane vesiculation leading to shedding of lipid particles that promote coagulation. Endothelial cells lost the cell surface heparin sulfate proteoglycans that normally interact with antithrombin III to inhibit coagulation. These processes contribute to thrombosis and vascular occlusion and the grafted organ suffers irreversible ischemic damage. When a graft is applied to an animal possessing the specific antibodies in high titres, hyperacuterejection takes place. The graft remains pale and is rejected within hours even without an attempt at vascularization. This was known as the white graft response. Humoral antibodies may sometimes act in opposition to cell mediated immunity, by inhibiting graft rejection. This phenomenon called immunological enhancement. It was originally described by kaliss in tumor transplants.
Acute Rejection:
Acute rejection is a process of vascular and parenchymal injury mediated by T cells and antibodies that usually begins after the fist week of transplantation. Effector T cells and antibodies that mediate acute rejection develop during a few days or weeks in response to the graft, accounting for the time at onset of acute rejection. The activated T cells cause direct lysis of graft cells or produce cytokines that recruit and activate inflammatory cells, which injure the graft. In vascualrized grafts such as kidney grafts, endothelial cells are the earliest targets of acute rejection. Microvascular endothelitis is a frequent early finding in acute rejection episodes. Endothelitis or intimal arteritis in medium-sized arteries also occurs at an early stage of acute rejection and is indicative of severe rejection, which left untreated, will likely result in acute graft failure. Antibodies can also mediate acute rejection if a graft recipient mounts a humoral immune response to vessel wall antigens and the antibodies that are produced bind to the vessel wall and activate complement. The histologic pattern of this form of acute rejection is one of transmural necrosis of graft vessel walls with acute inflammation, which is different from the thrombotic occlusion without vessel wall necrosis seen in Hyperacute rejection.
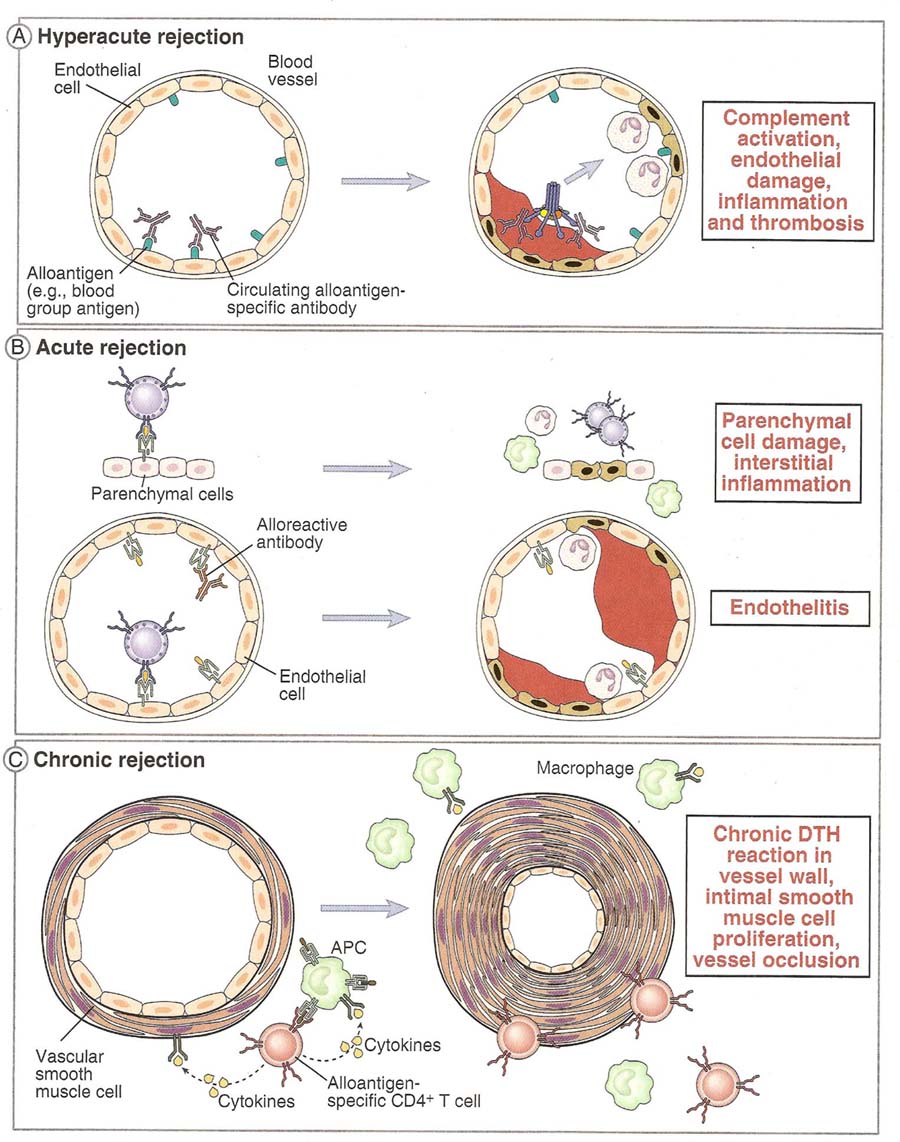
Chronic Rejection:
Chronic rejection is characterized by fibrosis and vascular abnormalities with loss of graft function occurring during a prolonged period. The firbrosis of chronic rejection may result from immune reactions and the production of cytokines that stimulate fibroblasts, or it may represent would heal after parenchymal cellular necrosis of acute rejection. Perhaps the major cause of chronic rejection of vascularised organ grafts is arterial occlusion as a result of the proliferation of intimal smooth muscle cells. This process called accelerated or graft arteriosclerosis. Graft arteriosclerosis is frequently seen in failed cardiac and renal allografts and can develop in any vascularlized organ transplant within 6 months to a year after transplantation. Chronic rejection of different transplanted organs is associated with distinct pathologic changes. Lung transplants undergoing chronic rejection show thickened small airways and liver transplants show fibrotic and nonfunctional bile ducts.
MECHANISM OF GRAFT REJECTION:
Graft rejection is caused principally by a cell-mediated immune response to alloantigens primarily, MHC molecules) expressed on cells of the graft. Both delayed-type hypersensitive and cell-mediated cytotoxicity reactions have been implicated. The process of graft rejection can be divided into two stages: (1) a sensitization phase, in which antigen-reactive lymphocytes of the recipient proliferate in response to alloantigens on the graft, and (2) an effector stage, in which immune destruction of the graft takes place.
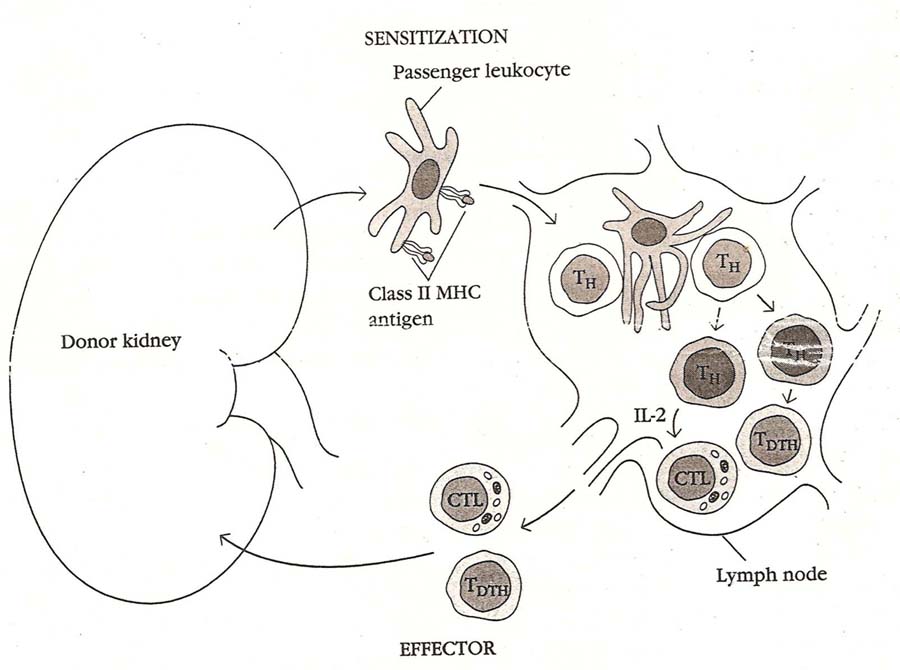
SENSITIZATION STAGE
During the sensitization phase, CD4+ and CD8+ T cells recognize alloantigens expressed on cells of the foreign graft and proliferate in response. Both major and minor histocompatibility alloantigens can be recognized. In general, the response to minor histocompatibility antigens is weak, although the combined response to several minor differences can sometimes be quite vigorous. The response to major histocompatibility antigens involves recognition of both the donor MHC molecule and an associated peptide ligand in the cleft of the MHC molecule. The peptides present in the groove of allogenic class I MHC molecules are derived from proteins synthesized within the allogenic cell. The peptides present in the groove of allogenic class II MHC molecules are generally proteins taken up and processed through the endocytic pathway of the allogenic antigen-presenting cell. A host TH cell becomes activated when it interacts with an antigen-presenting cell (APC) that both expresses an appropriate antigenic ligand–MHC molecule complex and provides the requisite co-stimulatory signal. Depending on the tissue, different populations of cells within a graft may function as APCs. Because dendritic cells are found in most tissues and because they constitutively express high levels of class II MHC molecules, dendritic cells generally serve as the major APC in grafts. APCs of host origin can also migrate into a graft and endocytose the foreign alloantigens (both major and minor histocompatibility molecules) and present them as processed peptides together with self-MHC molecules. In some organ and tissue grafts (e.g., grafts of kidney, thymus, and pancreatic islets), a population of donor APCs called passenger leukocytes has been shown to migrate from the graft to the regional lymph nodes. These passenger leukocytes are dendritic cells, which express high levels of class II MHC molecules (together with normal levels of class I MHC molecules) and are widespread in mammalian tissues, with the chief exception of the brain. Because passenger leukocytes express the allogeneic MHC antigens of the donor graft, they are recognized as foreign and therefore can stimulate immune activation of T lymphocytes in the lymph node. Recognition of the alloantigens expressed on the cells of a graft induces vigorous T-cell proliferation in the host. This proliferation can be demonstrated in vitro in a mixedlymphocyte reaction. Both dendritic cells and vascular endothelial cells from an allogeneic graft induce host T-cell proliferation. The major proliferating cell is the CD4+ T cell, which recognizes class II alloantigens directly or alloantigen peptides presented by host antigen-presenting cells. This amplified population of activated TH cells is thought to play a central role in inducing the various effector mechanisms of allograft rejection.
EFFECTOR STAGE
A variety of effector mechanisms participate in allograft rejection. The most common are cell-mediated reactions involving delayed-type hypersensitivity and CTL mediated cytotoxicity; less common mechanisms are antibodyplus- complement lysis and destruction by antibody-dependent cell-mediated cytotoxicity (ADCC). The hallmark of graft rejection involving cell-mediated reactions is an influx of T cells and macrophages into the graft. Histologically, the infiltration in many cases resembles that seen during a delayed type hypersensitive response, in which cytokines produced by TDTH cells promote macrophage infiltration. Recognition of foreign class I alloantigens on the graft by host CD8+ cells can lead to CTL-mediated killing. In some cases, CD4+ T cells that function as class II MHC–restricted cytotoxic cells mediate graft rejection.
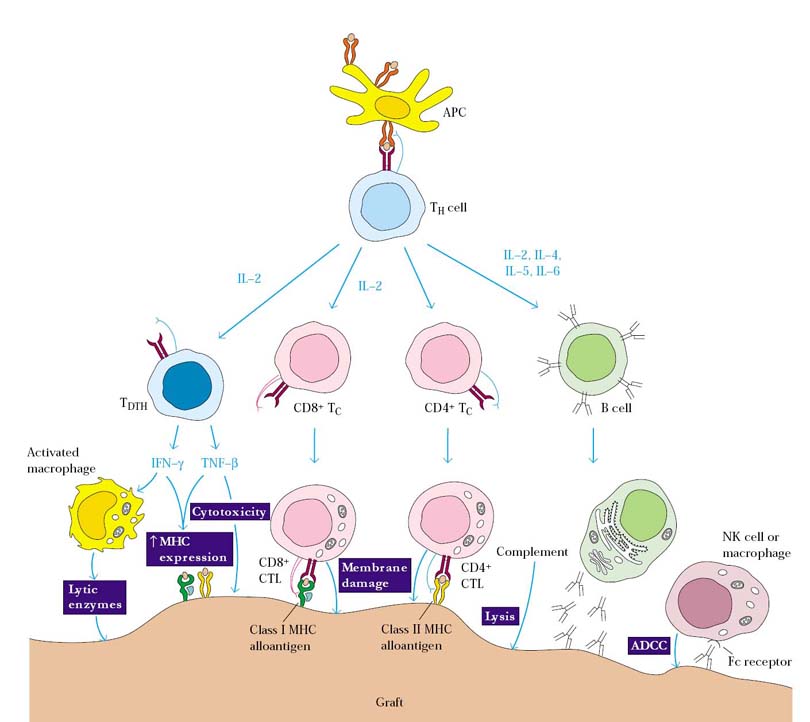
In each of these effector mechanisms, cytokines secreted by TH cells play a central role. For example, IL-2, IFN-g, and TNF-b have each been shown to be important mediators of graft rejection. IL-2 promotes T-cell proliferation and generally is necessary for the generation of effector CTLs. IFN-g is central to the development of a DTH response, promoting the influx of macrophages into the graft and their subsequent activation into more destructive cells. TNF-b has been shown to have a direct cytotoxic effect on the cells of a graft. A number of cytokines promote graft rejection by inducing expression of class I or class II MHC molecules on graft cells. The interferons (a, b, and g), TNF-a, and TNF-b all increase class I MHC expression, and IFN-g increases class II MHC expression as well. During a rejection episode, the levels of these cytokines increase, inducing a variety of cell types within the graft to express class I or class II MHC molecules. In rat cardiac allografts, for example, dendritic cells are initially the only cells that express class II MHC molecule. However, as an allograft reaction begins, localized production of IFN-g in the graft induces vascular endothelial cells and myocytes to express class II MHC molecules as well, making these cells targets for CTL attack.
Factors favoring allograft survival (Rejection Prevention):
Since difference in blood group and major histocompatibility antigens are responsible for the most intense graft rejection reactions, there are three main test has to be carried out before recommending transplant for transplantation. They are:
1. Blood grouping:
Initially, donor and recipient are screened for ABO blood-group compatibility. The blood-group antigens are expressed on RBCs, epithelial cells, and endothelial cells. Antibodies produced in the recipient to any of these antigens that are present on transplanted tissue will induce antibody mediated complement lysis of the incompatible donor cells. In order to determine ABO and Rh system, three drops of blood from finger tip is placed on slide. To these three drops, anti-A, anti-B and anti-D antibodies are placed respectively and mixed well. After some time, presence of agglutination checked out. Depending upon the appearance of agglutination, blood group identified as A positive, B negative or AB positive etc.

2. HLA typing:
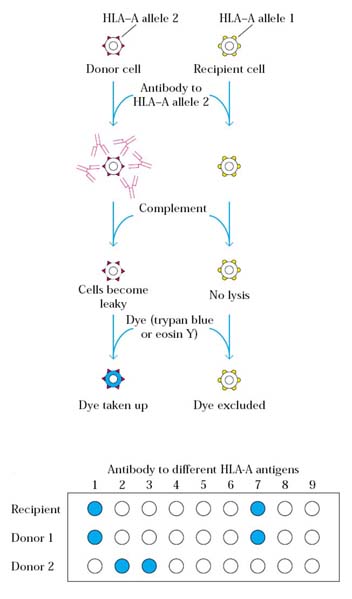
HLA typing of potential donors and a recipient can be accomplished with a microcytotoxicity test. In this test, white blood cells from the potential donors and recipient are distributed into a series of wells on a microtiter plate, and then antibodies specific for various class I and class II MHC alleles are added to different wells. After incubation, complement is added to the wells, and cytotoxicity is assessed by the uptake or exclusion of various dyes (e.g., trypan blue or eosin Y) by the cells. If the white blood cells express the MHC allele for which a particular monoclonal antibody is specific, then the cells will be lysed upon addition of complement, and these dead cells will take up a dye such as trypan blue. HLA typing based on antibody-mediated microcytotoxicity can thus indicate the presence or absence of various MHC alleles. It is not possible to get 100% compatible individuals. So that, more 60% compatibility was found to be good enough to recommend for transplantation. This achieved because of immunosuppressive therapy.
3. Tissue matching:
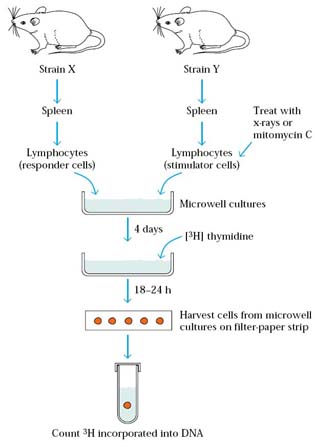
Tissue matching was tested with the help of Mixed Lymphocyte Reactions (MLR) and Cell Mediated Lympholysis (CML). MLR is an invitro system for assaying TH cell proliferation in a cell mediated response and CML is an invitro assay of effector cytotoxic function.
A one-way mixed-lymphocyte reaction (MLR) can be used to quantify the degree of class II MHC compatibility between potential donors and a recipient. Lymphocytes from a potential donor that have been x-irradiated or treated with mitomycin C serve as the stimulator cells, and lymphocytes from the recipient serve as responder cells. Proliferation of the recipient T cells, which indicates T-cell activation, is measured by the uptake of [3H]thymidine into cell DNA. The greater the class II MHC differences between the donor and recipient cells, the more [3H]thymidine uptake will be observed in an MLR assay. Intense proliferation of the recipient lymphocytes indicates a poor prognosis for graft survival. The advantage of the MLR over microcytotoxicity typing is that it gives a better indication of the degree of TH-cell activation generated in response to the class II MHC antigens of the potential graft. The disadvantage of the MLR is that it takes several days to run the assay. If the potential donor is a cadaver, for example, it is not possible to wait for the results of the MLR, because the organ must be used soon after removal from the cadaver. In that case, the microcytotoxicity test, which can be performed within a few hours, must be relied on.
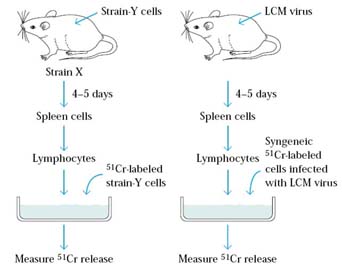
Development of the cell-mediated lympholysis (CML) assay was a major experimental advance that contributed to understanding of the mechanism of target-cell killing by CTLs. In this assay, suitable target cells are labeled intracellularly with chromium-51 (51Cr) by incubating the target cells with Na251CrO4. After the 51Cr diffuses into a cell, it binds to cytoplasmic proteins, reducing passive diffusion of the label out of the cell. When specifically activated CTLs are incubated for 1–4 h with such labeled target cells, the cells lyse and the 51Cr is released. The amount of 51Cr released correlates directly with the number of target cells lysed by the CTLs. By means of this assay, the specificity of CTLs for allogeneic cells, tumor cells, virus-infected cells, and chemically modified cells has been demonstrated.
IMMUNE RESPONSE IN GRAFT REJECTION:
Immune response in graft rejection are of two types namely host versus graft (HVG) reaction graft versus host (GVH) reaction.
Host versus Graft Reaction:
In most transplantation experiments, the graft tissue antigens in the case of allograft and xenograft induce an immune response in the host. This type of immune response is called as host versus graft reaction. Normal graft rejection mechanisms are of this type of reactions like first set rejection, Hyperacute, acute and chronic rejection and sensitized and effector phase rejections.
Graft versus Host Reaction:
When bone marrow transplanted with lymphocytes, the graft tissue elicits an immune response against the host antigens. This immune response is called graft versus host reaction.
The GVH reaction occurs when the following conditions are present:
Examples of situation leading to the GVH reactions are
a. allograft in a recipient in whom specific immunological tolerance has been induced.
b. adult lymphocytes injected into an immunologically deficient recipient. The immunological deficiency may be due to immaturity (newborn) or Immunosuppression and
c. F1 hybrid receiving a transplant from any one parental stain.
The major clinical features of the GVH reaction in animals are retardation of growth, emaciation, diarrhea, hepatospleenomegaly, lymphoid atrophy and anemia, terminating fatally. The syndrome has been called the runt disease.
Experimentally, GVH reactions develop when immunocompetent lymphocytes are transferred into an allogeneic neonatal or x-irradiated animal. The recipients, especially neonatal ones, often exhibit weight loss. The grafted lymphocytes generally are carried to a number of organs, including the spleen, where they begin to proliferate in response to the allogeneic MHC antigens of the host. This proliferation induces an influx of host cells and results in visible spleen enlargement, or splenomegaly. The intensity of a GVH reaction can be assessed by calculating the spleen index as follows:
Weight of experimental spleen/total body weight
Spleen index = ------------------------------------------------------------
Weight of control spleen/total body weight
A spleen index of 1.3 or greater is considered to be indicative of a positive GVH reaction. Spleen enlargement results from proliferation of both CD4+ and CD8+ T-cell populations. NK cells also have been shown to play a role in the GVH reaction, and these cells may contribute to some of the skin lesions and intestinal-wall damage observed.
IMMUNOLOGICALLY PRIVILEGED SITES:
There are certain sites in the body, called immunologically privileged sites, where an allograft can be placed without engendering a rejection reaction. These sites include the anterior chamber of the eye, the cornea, the uterus, the testes, and the brain. The cheek pouch of the Syrian hamster is a privileged site used in experimental situations. Each of these sites is characterized by an absence of lymphatic vessels and in some cases by an absence of blood vessels as well. Consequently, the alloantigens of the graft are not able to sensitize the recipient’s lymphocytes, and the graft has an increased likelihood of acceptance even when HLA antigens are not matched. The privileged location of the cornea has allowed corneal transplants to be highly successful. The brain is an immunologically privileged site because the blood-brain barrier prevents the entry or exit of many molecules, including antibodies. The successful transplantation of allogeneic pancreatic islet cells into the thymus in a rat model of diabetes suggests that the thymus may also be an immunologically privileged site. Immunologically privileged sites fail to induce an immune response because they are effectively sequestered from the cells of the immune system. This suggests the possibility of physically sequestering grafted cells. In one study, pancreatic islet cells were encapsulated in semipermeable membranes (fabricated from an acrylic copolymer) and then transplanted into diabetic mice. The islet cells survived and produced insulin. The transplanted cells were not rejected, because the recipient’s immune cells could not penetrate the membrane. This novel transplant method enabled the diabetic mice to produce normal levels of insulin and may have application for treatment of human diabetics.
XENOTRANSPLANTATION:
Transfer of xenograft is referred as xenotransplantation. Primates have served as the main transplant donors, with the earliest xenotransplants of chimpanzee kidney into humans dating to 1964. To date kidney, heart, liver and bone marrow xenotransplants from primates into humans have been performed. A major concern with xenotransplantation is the potential for the spread of pathogens from the donor to the recipient. These disease called as xenozoonoses, could potentially cause deadly infections in humans. A number of viral diseases, of limited pathogenicity in primates, have been shown to cause deadly infections in humans.
IMMUNOSUPPRESSIVE THERAPY:
Both Non-specific and Specific immunosuppressive therapies were provided to transplanted individuals in order to prevent allograft rejection.