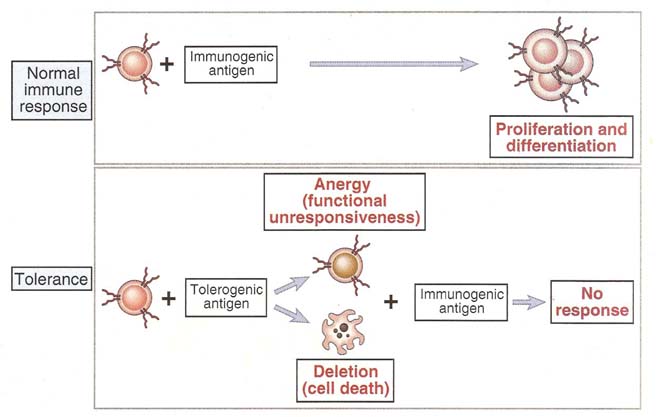
IMMUNOLOGICAL TOLERANCE
Immunologic tolerance or immunological unresponsiveness is the unresponsiveness of the adaptive immunity to antigens, as a result of inactivation or death of antigen specific lymphocytes, induced by exposure to antigens. Tolerance to self antigens is normal feature of the adaptive immune system, but tolerance to foreign antigens may be induced under certain conditions like hypersensitivity or transplantations.
An antigen which induces immune tolerance is known as tolerogens to distinguish from immunogens, which generate immunity. Many antigens can be either tolerogens or immunogens, depending upon how they are administered. Tolerogenic forms of antigens include large dose of the proteins administered without adjuvants, orally administered antigens and antigen presenting cells with low levels of costimulators and cytokines.
The first example of immunological tolerance was the observation by Owen of erythrocyte chimerism in dizygotic cattle twins, each of the twins having erythrocytes of its own and the otherís blood groups. As dizygotic twins are genetically dissimilar, they do not ordinarily accept transplants from each other but such transplants survive in cattle twins. The reason for this tolerance was shown to be the sharing of the same placental blood supply by the twins during intrauterine life. Based on this observation, Burnet and Fenner suggested that the unresponsiveness of individuals to self antigens was due to the contact of the immature immunological system with self antigens during embryonic life. Any antigen that comes into contact with the immunological system during embryonic life would be recognized as a self antigen and would not induce any immune response. They postulated that tolerance could be induced against foreign antigens if they were administered during embryonic life. This was proved experimentally by Medawar and his colleagues using two strains of syngenic mice. When skin graft from one inbred strain of mice (CBA) is applied on a mouse of another strain (A), it is rejected. If CBA cells are injected into fetal or newborn A strain mice. However, the latter when they grow up will freely accept skin grafts from CBA mice. The content of the self antigen appears to have been enlarged by contact with a foreign antigen during embryonic life. This phenomenon is called specific immunological tolerance.
With certain antigens, tolerance can be induced by two types of doses, one high and the other low, with intermediate doses producing immunity instead of tolerance. These are known as high zone and low zone tolerance. Tolerance can be overcome spontaneously or by an injection of cross reacting immunogens. For example, tolerance to bovine serum albumin in rabbits can be abolished by immunization with cross reacting human serum albumin. When immune unresponsiveness is established for one parameter of other immune responses and not to the other, then it is known as split tolerance. In guinea pigs, delayed type hypersensitivity to tuberculin can be inhibited, without affecting the production of a circulating antibody, by the injection of tuberculoprotein prior to immunization with BCG.
SELF TOLERANCE:
Immunological tolerance against self antigen was known as self tolerance. Self tolerance may be induced in generative lymphoid organs as a sequence of immature self reactive lymphocytes recognizing self antigens, called central tolerance or in peripheral sites as a result of mature self reactive lymphocytes encountering self antigens under particular conditions, called peripheral tolerance.
It is a fundamental property of normal immune system. All individuals inherit essentially the same antigen receptors genes and these genes recombine and are expressed in lymphocytes as the lymphocytes arise from stem cells. The molecular mechanism that generate functional antigen receptor genes from germ line sequences are random and are not influenced by foreign or self antigen for each individual. As a result, different clones of immature lymphocytes may express receptors capable of recognizing various foreign antigens as well as self antigens. Potentially immunogenic self antigens are present on the cells and in the circulation of every individual. Individual lymphocytes may have free access to many of these self antigens yet they normal do not mount immune responses against the antigens. This is because of self tolerance.

Self tolerance is maintained by several different mechanisms that prevent the maturation and activation of potentially harmful self reactive lymphocytes. It is due to self/non-self discrimination i.e the ability of the normal immune to recognize and respond to foreign antigen but not to self antigen. Failure of self tolerance results in immune reactions against self antigens. Such reactions are called as autoimmunity and the disease they cause are called as autoimmune disorders. Some self antigens may be ignored by the immune system, so that lymphocytes encounter the self antigen but fail to respond in any defectable way and remain viable and functional.
Foreign antigens may be administered in ways that inhibit immune responses by inducing tolerance in specific lymphocytes. The induction of immune tolerance may be exploited as a therapeutic approach for preventing harmful immune responses.
MECHANISM:
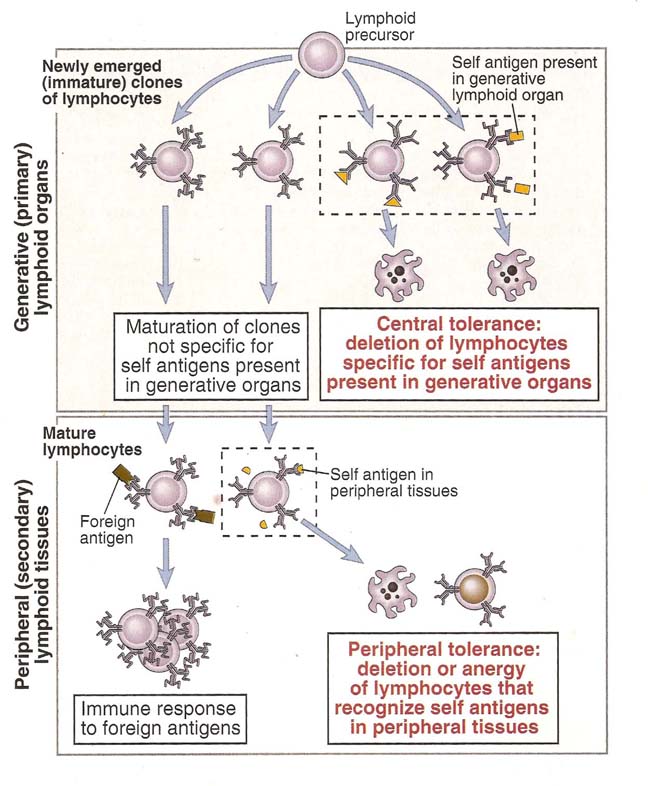
Tolerance is immunologically specific and results from the recognition of antigens by specific lymphocytes. Self tolerance is of two types depending upon their place of occurrence namely central tolerance and peripheral tolerance.
Central tolerance occurs during their maturation in the generative lymphoid organs. All lymphocytes pass through a stage which encounter with antigen leads to tolerance rather than activation. Usually only self antigens were available in high concentrations in generative lymphoid organs. Therefore, in the generative lymphoid organs, immature lymphocytes normally encounter only self antigens at high concentration and clones of lymphocytes whose receptors recognize these self antigens with high affinity are killed. This process is called as negative selection. This eliminates self reactive lymphocytes. Mostly T-cells obtain central tolerance.
Peripheral tolerance occurs as a result of self-reactive lymphocytes encountering self antigens under particular conditions. It is induced when mature lymphocytes recognize antigens without adequate levels of the costimulators that are required for activation or as a result of persistent and repeated stimulation by self antigens in peripheral tissues. It is the most important for maintaining unresponsiveness to self antigens that are expressed in peripheral tissues and not in generative lymphoid organs. Mostly B-cells obtain peripheral tolerance.
The principal mechanisms of lymphocyte tolerance are apoptotic cell death, called as clonal deletion; functional inactivation without cell death called clonal anergy; and suppression of lymphocytes activation and effector functions by regulatory lymphocytes. Central tolerance is mainly due to deletion, whereas all three mechanisms contribute to peripheral tolerance.
Depending upon the cells involved in Tolerance, it is classified into T-cell tolerance and B-cell tolerance.
T-cell Tolerance:
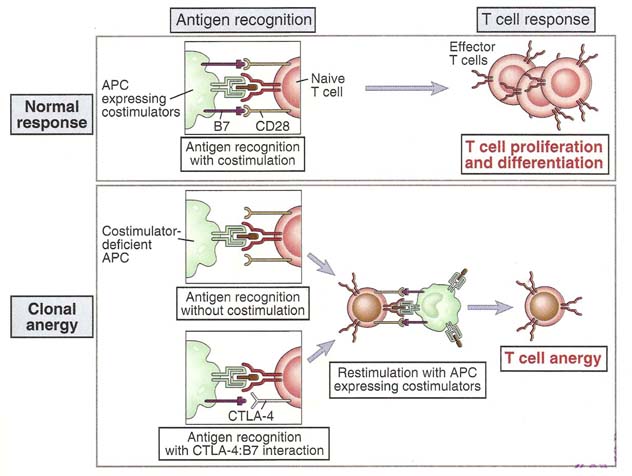
In T-lymphocytes, central tolerance through negative selection occurs when double positive lymphocytes with high affinity receptors for self antigen recognize these antigens in the thymus. Several mechanisms account for peripheral tolerance in mature T-cells. In CD4+ T-cells, anergy is induced by antigen recognition without adequate costimulation and by recognition of altered forms of the native antigen. Repeated stimulation of T-cells by persistant antigens results in activation induced cell death. Some self antigens activate regulator T-cells, which inhibit immune responses in part by producing, immunosuppressive cytokines.
B-cell Tolerance:
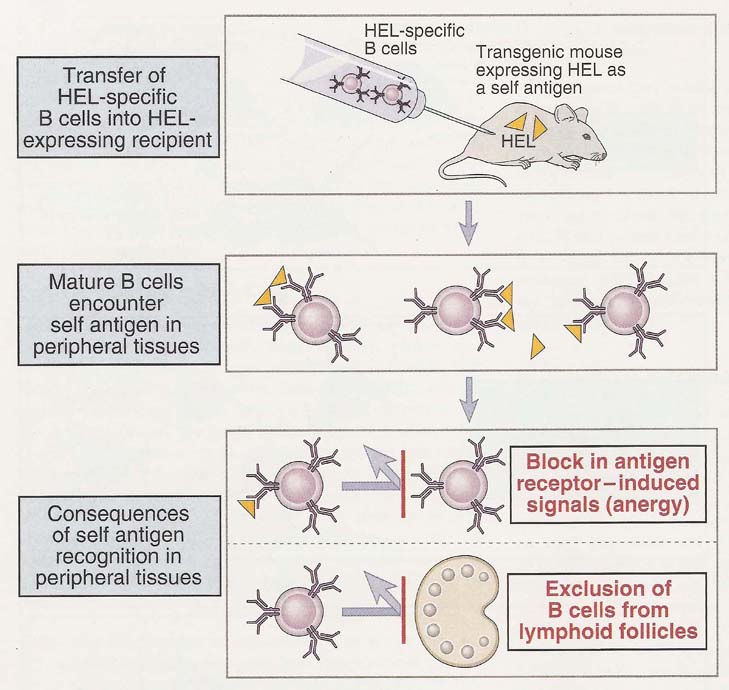
In B-lymphocytes, central tolerance is induced when immature B-cells recognize multivalent self antigens in the bone marrow. The usual result is apoptotic death of the B-cells or due to the acquisition of new specificity called receptor editing. Mature B-lymphocytes that recognize self antigens in peripheral tissues in the absence of specific T-helper cells may be rendered functionally unresponsive or are excluded from lymphoid follicles and cannot be activated by antigens and result in B-cell tolerance.
Tolerance against Foreign Proteins:
Foreign antigens may be administered in ways that preferentially induce tolerance rather than immune responses. In general, protein antigens administered with adjuvants favor immunity whereas high doses of antigens administered systemically without adjuvants tend to induce tolerance. The likely reason for this is that adjuvants stimulate the expression of costimulators on APCs and in the absence of costimulation, T-cells that recognize the antigen may become anergic. Tolerogenic antigens may also activate regulatory T-cells or promote the differentiation of suppressor T-cells into populations that produce cytokines such as IL-4 which does not induce cell mediated immunity. The oral administration of a protein antigen often leads to a marked suppression of systemic humoral and cell mediated immune responses to immunization with the same antigen. This phenomenon is called as oral tolerance. It has been postulated that the physiologic importance of oral tolerance may be as a mechanism for preventing immune responses to food antigens and to bacteria that normally reside as commensals in the intestinal lumen and are needed for digestion and absorption.
Factors favoring tolerance and immune response depicted in the table:
|
S.No |
Factors |
Favoring Immune Response |
Favoring Tolerance |
|
1. |
Amount |
Optimal dose that vary for different antigens |
High doses |
|
2. |
Persistence |
Short-lived |
Prolonged |
|
3. |
Portal entry and Location |
Subcutaneous, intradermal and absence from generative organs. |
Intravenous, oral and presence in generative organs |
|
4. |
Presence of adjuvants |
Antigens with adjuvants |
Antigens without adjuvants |
|
5. |
Properties of APC |
High levels of costimulators |
Low levels of co stimulators and cytokines |
Tolerance against allergens induced through the process of hyposensitization which prevents type I hypersensitivity. Similarly, tolerance against allograft was achieved by subjecting recipient to immunosuppressive treatment for short period of time after transplantation. These are few examples where tolerance against foreign antigen used as positive mechanisms.
IMMUNOS SUPPRESSION
Inhibition of one or more components of the adaptive or innate immune system as a result of an underlying disease or intentionally induced by drugs for the purpose of preventing or treating graft rejection or autoimmune disease.
Immunosuppression found to play vital role in transplantation, hypersensitivity reactions, autoimmune diseases and inflammatory reactions. Because of Immunosuppression therapy, cancers and fungal infections might be aroused as secondary level of diseases.
Immunosuppressive agents might be classified into two broad headings namely Non-specific and Specific Immunosuppressive agents.
NON-SPECIFIC IMMUNOSUPPRESSION:
The non-specificity refers to the state in which Immunosuppression achieved by blocking polyclone of lymphocytes. Under this category, there are five components involved namely mitotic inhibitors, Steroidal agents, Non-steroidal anti-inflammatory agents, chemotherapeutic agents and lymphoid irradiation.
Mitotic Inhibitors:
Not all types of mitotic inhibitors used under this category. Only those possess inhibitory mechanisms against lymphocytes are used. Azathioprine (Imuran), a potent mitotic inhibitor, is diminish T-cell and B-cell proliferation in response to antigens which might be either allogens or autogens. It acts on cells in the S-phase of the cell cycle to block synthesis of inosinic acid, which is a precursor of the purines adenylic and guanylic acid. The other two mitotic inhibitors that are sometimes used in conjunction with other immunosuppressive agents are cyclophosphoamide and methotrexate. Cyclophosphamide is an alkylating agent that inserts into the DNA helix and becomes cross-linked, leading to disruption of the DNA chain. It is especially effective against rapidly dividing cells and therefore is sometimes given at the time of grafting to block T-cell proliferation. Methotrexate acts a folic acid antagonist to block dihydrofolate reducatase enzyme which intern blocks purine biosynthesis.
Mycopheonolate mofetil (MMF) is metabolized to mycophenolic acid, which blocks a lymphocyte-specific isoform of inosine monophosphate dehydroxygenase, an enzyme required for denovo synthesis of guanine nucleotides. Because MMF selectively inhibits the lymphocyte-specific isoform of this enzyme, it has relatively few toxic effects. MMF is now routinely used in combination with cyclosporine to prevent acute allograft rejection.
CORTICOSTEROIDS:
The corticosteroids, which are cholesterol derivatives, include prednisone, prednosolone and methylprednisolone. Like other steroid hormones, the corticosteroids are lipophilic and thus can cross the plasma membrane and bind to receptors in the cytosol. The resulting receptor-hormone complexes are subsequently transported to the nucleus where they bind to specific regulatory DNA sequences, either up-regulating or down-regulating transcription. The corticosteroids have been shown to induce increased transcription of the NR-kB inhibitor (IKB). Binding of this inhibitor to NF-kB in the cytosol prevents the translocation of NF-kB into the nucleus and consequently prevents NF-kB activation of a number of genes, including genes involved in T-cell activation and cytokine production.
Corticosteroids also reduce both phagocytic and killing ability of macrophages and neutrophils. The proposed mechanism of action for these natural hormones and their synthetic analogues is to block the synthesis and secretion of cytokines, including tumor necrosis factor (TNF) and IL-1, by macrophages. Lack of TNF and IL-1 synthesis reduces graft endothelial cell activation and recruitment of inflammatory leukocytes. Corticosteroids may also block other effectors mechanisms of macrophages, such as the generation of prostaglandins, reactive oxygen intermediates, and nitric oxide. They also reduce the expression of MHC-II molecules on the surface of macrophages which intern reduce the activity of macrophages as APC. Corticosteroids also stabilize the lysosomal membrane, so that decreased levels of lysosomal enzymes are released at the site of inflammation.
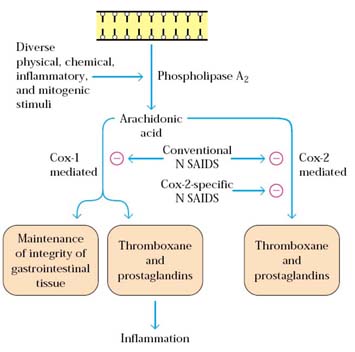
NON-STEROIDAL ANTIINFLAMMATORY DRUGS (NSAIDs):
NSAIDs are the most frequently used medication for treating pain and inflammation and also reduces immune responses to certain extent. One mechanism by which these drugs exert anti-inflammatory effects is by inhibiting the cyclooxygenase pathway by which prostaglandins and thromboxanes are produced from arachidonic acid. The reduction in prostaglandin production limits the increase in vascular permeability and neutrophil chemotaxis in the inflammatory response. Clinically, NSAIDs have been shown to be effective for treatment of many acute and chronic inflammatory reactions. Asprin which contain salicylate act as one of common NSAIDs.
CHEMOTHERAPEUTIC AGENTS:
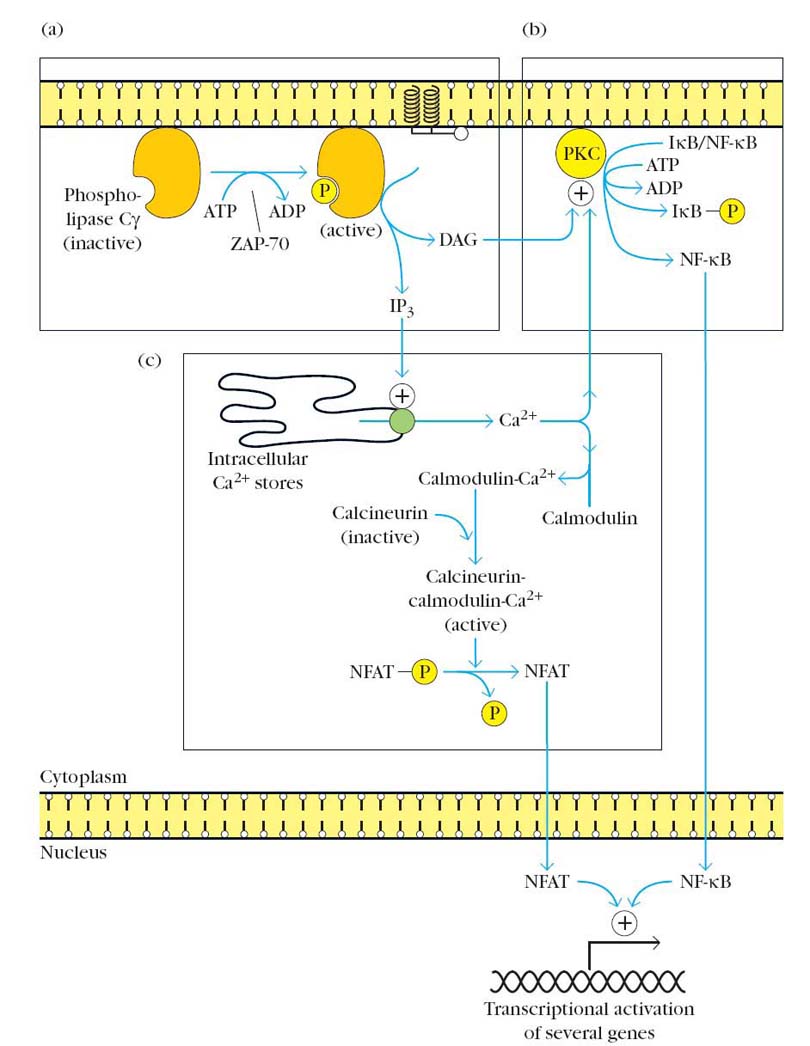
Cyclosporin A (CsA), FK506 and rapamycin are fungal metabolites with potent immunosuppressive properites. The major action of Cyclosporine on T cells is to inhibit the transcription of certain genes most notably those encoding cytokines such as interleukin -2. Cyclosporine binds with high affinity to ubiquitous cellular protein called cyclophilin which commonly known as immunophilin.
The complex of cyclosporine and cyclophilin binds to and inhibits the enzymatic activity of the calcium/calmodulin activated protein phosphatase calcineurin. Because calcium/calmodulin activated calcineurin function is required to activate the transcription factor NFAT (Nuclear Factor of activated T cells), cyclosporine blocks NFAT activation and the transcription of IL-2 and other cytokine genes. The net result is that cyclosporine blocks the IL-2 dependent growth and differentiation of T cells. Even though FK506 structurally different from cyclosporine, it binds to immunophilin known as FKBP and exert same function like cyclosporine.
Like FK506, rapamycin also binds to FKBP, but the rapamycin-FKBP complex does not inhibit calcineurin. Instead, this complex binds to another cellular protein called the mammalian target of rapamycin (MTOR). The mechanism by which rapamycin inhibits T cell growth is not known. Rapamycin-treated T-cell display abnormalities in the levels of certain cyclin/cyclin-dependent kinase complexes, suggesting that MTOR may be a regulator of a protein kinase that participates in cell cycle control. Combinations of cyclosporine and rapamycin are potent inhibitors of T-cell response. Brequinar, is another antibiotic which is used to inhibit antibody synthesis and are therefore potentially useful in preventing acute vascular rejection of transplantation.
LYMPHOCYTE IRRADIATION:
Because lymphocytes are extremely sensitive to x-rays, x-irradiation can be used to reduce functional lymphocytes. The typical protocol involves daily x-irradiation treatment of about 200 rads per day for several weeks until a total of 3400 rads has been administered. Thymus, spleen and lymph nodes usually subjected to x-ray irradiation. Bone marrow not x-irradiated, so that it can produce new lymphocytes after certain period of time. This type of Immunosuppression plays vital role in transplantations.
SPECIFIC IMMUNOSUPPRESSIVE THERAPY:
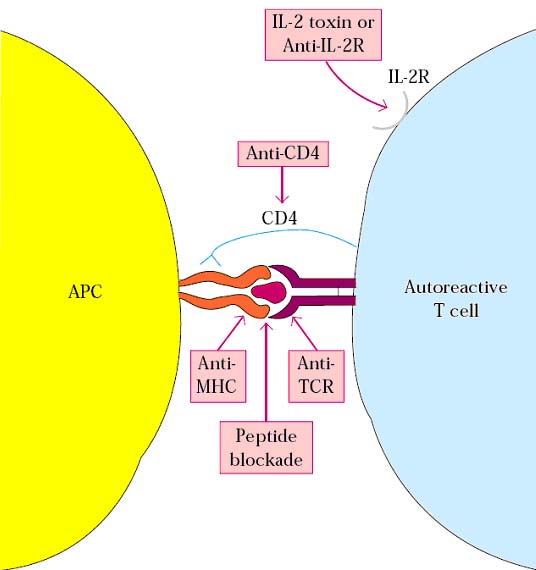
Specific immunosuppressive therapy is specifically used to suppress immune response against specific epitopes and antigens. Specific Immunosuppression is achieved mainly with the help of monoclonal antibodies. Monoclonal antibodies against T-cell surface structures, co-stimulator signals and cytokines were used to block immune response against specific antigens thus this type of Immunosuppression is also otherwise known blocking method.
Antigenic shift: It is defined as sudden emergence of a new pathogen subtype possibly resulting from genetic reassortment leading to substantial antigenic differences. It is easily recognized by antibodies and it induces the production of different antibodies.
Antigenic drift: It is defined as a series of spontaneous point mutations that generate minor antigenic variations in pathogens and lead to strain differences. It usually not recognized by antibodies or in otherwise, antigenic drift does not induce production of different antibodies.
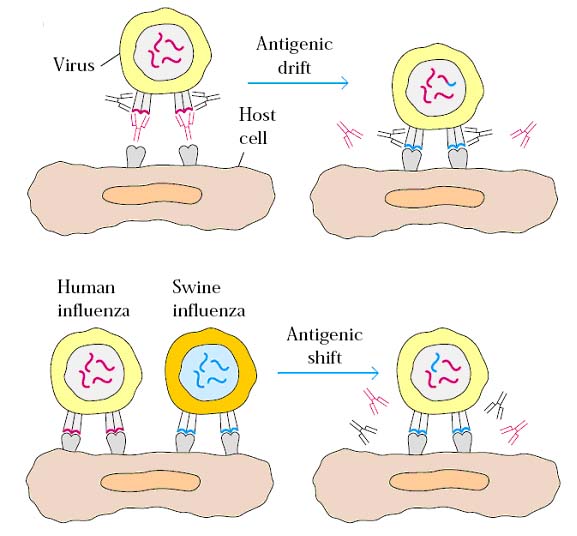
Both the events play vital role in antigenic variation of infecting agents such as viruses. Each antigenic shift finds the population immunologically unprepared, resulting in major outbreaks whereas antigenic drift generating minor antigenic variations which account for stain differences.
ANAMNESTIC RESPONSE:
Elevated immune response to an antigen exposed for the second time
caused by memory cell. Secondary Immune response is otherwise known as
anamnestic response.