
IMMUNIZATION
Immunization, also called vaccination or inoculation, a method of stimulating resistance in the human body to specific diseases using microorganisms—bacteria or viruses—that have been modified or killed. These treated microorganisms do not cause the disease, but rather trigger the body's immune system to build a defense mechanism that continuously guards against the disease. If a person immunized against a particular disease later comes into contact with the disease-causing agent, the immune system is immediately able to respond defensively.
Immunization has dramatically reduced the incidence of a number of deadly diseases. For example, a worldwide vaccination program resulted in the global eradication of smallpox in 1980, and in most developed countries immunization has essentially eliminated diphtheria, poliomyelitis, and neonatal tetanus. The number of cases of Haemophilus influenzae type b meningitis in the United States has dropped 95 percent among infants and children since 1988, when the vaccine for that disease was first introduced. In the United States, more than 90 percent of children receive all the recommended vaccinations by their second birthday. About 85 percent of Canadian children are immunized by age two.
HISTORY OF IMMUNIZATION
The use of immunization to prevent disease predated the knowledge of both infection and immunology. In China in approximately 600 BC, smallpox material as inoculated through the nostrils. Inoculation of healthy people with a tiny amount of material from smallpox sores was first attempted in England in 1718 and later in America. Those who survived the inoculation became immune to smallpox. American statesman Thomas Jefferson traveled from his home in Virginia to Philadelphia, Pennsylvania, to undergo this risky procedure. This technique is referred as Variolation.
In 1718 Lady Mary Wortley Montagu, the wife of the British ambassador to Constantinople, observed the positive effects of variolation on the native population and had the technique to her own children. This technique was significantly improved by the Edward Jenner. A significant breakthrough came in 1796 when British physician Edward Jenner discovered that he could immunize patients against smallpox by inoculating them with material from cowpox sores. Cowpox is a far milder disease that, unlike smallpox, carries little risk of death or disfigurement. Jenner inserted matter from cowpox sores into cuts he made on the arm of a healthy eight-year-old boy. The boy caught cowpox. However, when Jenner exposed the boy to smallpox eight weeks later, the child did not contract the disease. The vaccination with cowpox had made him immune to the smallpox virus. Today we know that the cowpox virus antigens are so similar to those of the smallpox virus that they trigger the body's defenses against both diseases. In 1885 Louis Pasteur created the first successful vaccine against rabies for a young boy who had been bitten 14 times by a rabid dog. Over the course of ten days, Pasteur injected progressively more virulent rabies organisms into the boy, causing the boy to develop immunity in time to avert death from this disease. Another major milestone in the use of vaccination to prevent disease occurred with the efforts of two American physician-researchers. In 1954 Jonas Salk introduced an injectable vaccine containing an inactivated virus to counter the epidemic of poliomyelitis. Subsequently, Albert Sabin made great strides in the fight against this paralyzing disease by developing an oral vaccine containing a live weakened virus. Since the introduction of the polio vaccine, the disease has been nearly eliminated in many parts of the world.
As more vaccines are developed, a new generation of combined vaccines are becoming available that will allow physicians to administer a single shot for multiple diseases. Work is also under way to develop additional orally administered vaccines and vaccines for sexually transmitted infections. Possible future vaccines may include, for example, one that would temporarily prevent pregnancy. Such a vaccine would still operate by stimulating the immune system to recognize and attack antigens, but in this case the antigens would be those of the hormones that are necessary for pregnancy.
TYPES OF IMMUNIZATION
Scientists have developed two approaches to immunization: active immunization, which provides long-lasting immunity, and passive immunization, which gives temporary immunity. In active immunization, all or part of a disease-causing microorganism or a modified product of that microorganism is injected into the body to make the immune system respond defensively. Passive immunity is accomplished by injecting blood from an actively immunized human being or animal.
Passive Immunization
Passive immunization is performed without injecting any antigen. In this method, vaccines contain antibodies obtained from the blood of an actively immunized human being or animal. The antibodies last for two to three weeks, and during that time the person is protected against the disease. Although short-lived, passive immunization provides immediate protection, unlike active immunization, which can take weeks to develop. Consequently, passive immunization can be lifesaving when a person has been infected with a deadly organism.
Occasionally there are complications associated with passive immunization. Diseases such as botulism and rabies once posed a particular problem. Immunoglobulin (antibody-containing plasma) for these diseases was once derived from the blood serum of horses. Although this animal material was specially treated before administration to humans, serious allergic reactions were common. Today, human-derived immune globulin is more widely available and the risk of side effects is reduced.
Active Immunization:
Vaccines that provide active immunization are made in a variety of ways, depending on the type of disease and the organism that causes it. The active components of the vaccinations are antigens, substances found in the disease-causing organism that the immune system recognizes as foreign. In response to the antigen, the immune system develops either antibodies or white blood cells called T lymphocytes, which are special attacker cells. Immunization mimics real infection but presents little or no risk to the recipient. Some immunizing agents provide complete protection against a disease for life. Other agents provide partial protection, meaning that the immunized person can contract the disease, but in a less severe form. These vaccines are usually considered risky for people who have a damaged immune system, such as those infected with the virus that causes acquired immunodeficiency syndrome (AIDS) or those receiving chemotherapy for cancer or organ transplantation. Without a healthy defense system to fight infection, these people may develop the disease that the vaccine is trying to prevent. Some immunizing agents require repeated inoculations—or booster shots—at specific intervals.
Tetanus shots, for example, are recommended every ten years throughout life. In order to make a vaccine that confers active immunization, scientists use an organism or part of one that has been modified so that it has a low risk of causing illness but still triggers the body’s immune defenses against disease. One type of vaccine contains live organisms that have been attenuated—that is, their virulence has been weakened. This procedure is used to protect against yellow fever, measles, smallpox, and many other viral diseases. Immunization can also occur when a person receives an injection of killed or inactivated organisms that are relatively harmless but that still contain antigens. This type of vaccination is used to protect against bacterial diseases such as poliomyelitis, typhoid fever, and diphtheria.
Some vaccines use only parts of an infectious organism that contain antigens, such as a protein cell wall or a flagellum. Known as acellular vaccines, they produce the desired immunity with a lower risk of producing potentially harmful immune reactions that may result from exposure to other parts of the organism. Acellular vaccines include the Haemophilus influenzae type B vaccine for meningitis and newer versions of the whooping cough vaccine. Scientists use genetic engineering techniques to refine this approach further by isolating a gene or genes within an infectious organism that code for a particular antigen. The subunit vaccines produced by this method cannot cause disease and are safe to use in people who have an impaired immune system. Subunit vaccines for hepatitis B and pneumococcus infection, which causes pneumonia, became available in the late 1990s.
Active immunization can also be carried out using bacterial toxins that have been treated with chemicals so that they are no longer toxic, even though their antigens remain intact. This procedure uses the toxins produced by genetically engineered bacteria rather than the organism itself and is used in vaccinating against tetanus, botulism, and similar toxic diseases.
IMMUNIZATION RECOMMENDATIONS:
More than 50 vaccines for preventable diseases are licensed in the United States. The American Academy of Pediatrics and the U.S. Public Health Service recommend a series of immunizations beginning at birth. The initial series for children is complete by the time they reach the age of two, but booster vaccines are required for certain diseases, such as diphtheria and tetanus, in order to maintain adequate protection. When new vaccines are introduced, it is uncertain how long full protection will last. Recently, for example, it was discovered that a single injection of measles vaccine, first licensed in 1963 and administered to children at the age of 15 months, did not confer protection through adolescence and young adulthood. As a result, in the 1980s a series of measles epidemics occurred on college campuses throughout the United States among students who had been vaccinated as infants. To forestall future epidemics, health authorities now recommend that a booster dose of the measles, mumps, and rubella (also known as German measles) vaccine be administered at the time a child first enters school. Not only children but also adults can benefit from immunization. Many adults in the United States are not sufficiently protected against tetanus, diphtheria, measles, mumps, and German measles. Health authorities recommend that most adults 65 years of age and older, and those with respiratory illnesses, be immunized against influenza (yearly) and pneumococcus (once).

VACCINES:
Vaccines are classified into mainly four categories namely, Whole-Organism vaccines, Purified Macromolecules Vaccines, Recombinant Vaccines and Future Vaccines.
I. WHOLE-ORGANISM VACCINES
a. Live attenuated vaccines
b. Killed or inactivated vaccines
II. PURIFIED MACROMOLECULES
a. Toxoids
b. Capsular Polysaccharide vaccines
c. Polypeptide vaccines
III. RECOMBINANT VACCINES
a. Recombinant Protein vaccines
b. Recombinant vector vaccines
c. Subunit Vaccines
d. Polynucleotide vaccines (DNA vaccine)
IV FUTURE VAACINES
a. Multivalent subunit vaccine
b. Anti-idiotype vaccine
c. Plant vaccine
I. WHOLE-ORGANISM VACCINES:
a. Live attenuated vaccines:
Live attenuated vaccines usually are created from the naturally occurring germ itself. The germs used in these vaccines still can infect people, but they rarely cause serious disease. Viruses are weakened (or attenuated) by growing them over and over again in a laboratory under nourishing conditions called cell culture. The process of growing a virus repeatedly-also known as passing--serves to lessen the disease-causing ability of the virus. Vaccines are made from viruses whose disease-causing ability has deteriorated from multiple passages. For example vaccine for TB produced by Calmette-Guerin by growing Mycobacterium bovis in a culture medium rich in bile salts. It is an adverse condition for the bacteria. After 13 yrs, it was used as live attenuated vaccine.
Examples of live attenuated vaccines include:
· Measles vaccine (as found in the MMR vaccine)
· Mumps vaccine (MMR vaccine)
· Rubella (German measles) vaccine (MMR vaccine)
· Oral polio vaccine (OPV) (sabin vaccine)
· Varicella (chickenpox) vaccine
· BCG (Bacillus Calmette-Guerin ) vaccine for TB
|
Vaccine Type |
Bacterial Vaccines |
Viral Vaccines |
|
Live Attenuated Vaccine |
Tuberculosis – BCG, Typhoid Vaccine |
Measles Vaccine, Mumps Vaccine, Polio (sabin) Vaccine |
|
Killed Vaccine |
Anthrax, Cholera & Pertussis |
Rabies, Rubella & Polio (salk) Vaccine |
Several methods have been used to attenuate viruses for vaccine production.
Use of a related virus from another animal - the earliest example was the use of cowpox to prevent smallpox. The origin of the vaccinia viruses used for production is uncertain.
Administration of pathogenic or partially attenuated virus by an unnatural route - the virulence of the virus is often reduced when administered by an unnatural route. This principle is used in the immunization of military recruits against adult respiratory distress syndrome using enterically coated live adenovirus type 4, 7 and (21).
Passages of the virus in an “unnatural host” or host cell - The major vaccines used in man and animals have all been derived this way. After repeated passages, the virus is administered to the natural host. The initial passages are made in healthy animals or in primary cell cultures. There are several examples of this approach: the 17D strain of yellow fever was developed by passage in mice and then in chick embryos. Polioviruses were passaged in monkey kidney cells and measles in chick embryo fibroblasts. Human diploid cells are now widely used such as the WI-38 and MRC-5. The molecular basis for host range mutation is now beginning to be understood. To produce an attenuated virus, the virus must first be isolated by growing it in cultured human cells. The adaptation to growth in cultured human cells can cause some attenuation in itself; the rubella vaccine, for example, was made in this way. In general, however, the virus is then adapted to growth in cells of a different species, until it grows only poorly in human cells. The adaptation is a result of mutation, usually a combination of several point mutations. It is usually hard to tell which of the mutations in the genome of an attenuated viral stock are critical to attenuation. An attenuated virus will grow poorly in the human host, and will therefore produce immunity but not disease.
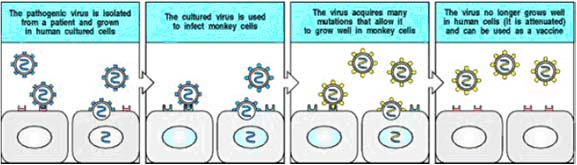
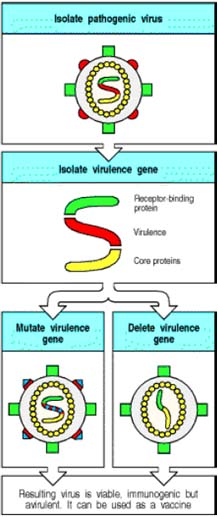
Development of temperature sensitive mutants - this method may be used in conjunction with the above method. Attenuation can be achieved more rapidly and reliably with recombinant DNA techniques. If a gene in the virus that is required for virulence but not for growth or immunogenicity can be identified, this gene can be either multiply mutated or deleted from the genome by using recombinant DNA techniques. This procedure creates an avirulent (nonpathogenic) virus that can be used as a vaccine. The mutations in the virulence gene are usually large, so that it is very difficult for the virus to revert to the wild type.
The Sabin oral polio vaccine and the measles, mumps, and rubella (MMR) vaccine are examples of attenuated (weakened) vaccines. To make an attenuated vaccine, the pathogen is grown in animals or tissue culture under conditions that make it less virulent. Advantages of whole virus attenuated vaccines are that infectious virus can stimulate generation of memory cellular as well as humoral immune responses; the ability of the virus to multiply in the host means that less virus must be injected to induce protection; and use of the whole virus stimulates response to antigens in their natural conformation. Additional advantages of the Sabin vaccine are that it can be administered orally, which is less expensive than giving injections; and oral administration induces mucosal immunity and IgA synthesis, which gives more protection at the normal site of virus entry. Disadvantages of attenuated vaccines are that the virus may very rarely revert to its virulent form and cause disease; and live virus vaccines cannot be given safely to immunosuppressed people. Because the incidence of vaccine-acquired polio is much higher than that of naturally acquired polio in the US, vaccination recommendations changed recently so that infants will receive killed polio vaccine prior to receiving the oral vaccine. The oral vaccine is being used in the WHO polio eradication campaign.
b. Inactivated (killed) vaccines:
Inactivated (killed) vaccines cannot cause an infection, but they still can stimulate a protective immune response. Viruses are inactivated with chemicals such as formaldehyde. In this vaccine, the replicating ability of the infecting agents is inhibited.
Examples of inactivated (killed) vaccines:
· Inactivated polio vaccine (IPV), which is the shot form of the polio vaccine
· Inactivated influenza vaccine
· Salk polio vaccine
· Pertussis vaccine used in DPT vaccine
The original Salk polio vaccine is an example of an inactivated (killed) vaccine. It is made by growing virulent polio virus in tissue culture, then treating the virus with formaldehyde so that it cannot reproduce in the person who receives the vaccine. Polio virus does not undergo rapid mutation, so a mixture of three serotypes of polio virus is sufficient to generate good protective immunity against all common polio viruses. Neutralizing antibody produced to polio virus is very efficient at blocking the ability of the virus to infect host cells and offers good protection from infection. The advantages of this vaccine are that the risk of infection is very low if inactivation is efficient; it can be used safely on people whose immune systems are compromised by chemotherapy for cancer, transplantation anti-rejection drugs, or other immune deficiencies; and use of the whole virus stimulates immunity to antigens in their natural conformation on the virus surface (essential for neutralizing antibodies). The disadvantages are that since the virus cannot multiply, a large number of virions are required to stimulate immunity; periodic boosters must be given to maintain immunity; only humoral immunity can be induced; and since the vaccine must be injected, it is costly to administer. Influenza virus vaccine is another inactivated virus vaccine. It has the additional disadvantage that flu viruses mutate rapidly, so that new antigenic specificities must be included in each year's vaccine. Predictions are made in the spring about which antigens will predominate the following year; if those predictions are wrong, the vaccine will not be protective.
|
S. No |
Features |
Live attenuated Vaccines |
Killed Vaccines |
|
1. |
Dose |
Low |
High |
|
2. |
Number of Doses |
Single |
Multiple (Booster) |
|
3. |
Need of Adjuvant |
No |
Yes |
|
4. |
Duration of Immunity |
Many years |
Less |
|
5. |
Antibody Response |
Ig G |
Ig G and Ig A |
|
6. |
Cell Mediated Response |
Good |
Poor |
|
7. |
Reversion to Virulence |
Possible |
Not Possible |
II. PURIFIED MACROMOLECULES VACCINES:
a. Toxoid vaccines:
Toxoid vaccines are made by treating toxins (or poisons) produced by germs with heat or chemicals, such as formalin, to destroy their ability to cause illness. Even though toxoids do not cause disease, they stimulate the body to produce protective immunity just like the germs' natural toxins.
Examples of toxoid vaccines:
· Diphtheria toxoid vaccine (may be given alone or as one of the components in the DTP, DTaP, or dT vaccines)
· Tetanus toxoid vaccine (may be given alone or as part of DTP, DTaP, or dT)
The diphtheria vaccine protects almost everyone who has received the full series of recommended doses. The number of people with diphtheria has fallen from 206,939 in 1921 to only a few a year in the United States.
b. Component vaccines (Purified Macromolecules as vaccine) (conjugated vaccine):
Some vaccines are made by using only parts of the viruses or bacteria. These vaccines cannot cause disease, but they can stimulate the body to produce an immune response that protects against infection with the whole germ. Four of the newest vaccines are made this way.
Examples of component vaccines:
· Haemophilus influenzae type b (Hib) vaccine
· Hepatitis B (Hep B) vaccine
· Hepatitis A (Hep A) vaccine
· Pneumococcal conjugate vaccine
The virulence of some pathogenic bacteria depends primarily on the antiphagocytic properties of their hydrophilic polysaccharide capsule. Coating of the capsule with antibodiesd and /or complement greatly increases the ability of macrophages and neutrophils to phagocytose such pathogens. These finding provide the rationale for vaccines consisting of purified capsular polysaccharides.
The current vaccine for Streptococcus pneumoniae, which causes pneumococcal pneumonia, consists of 23 antigenically different capsular polysaccharides. It is marketed as Pneumovax 23 by Merck and as Pneu-Immune 23 by Lederle Laboratories. The vaccine for Neisseria meningitides, a common cause of bacterial meningitis, also consists of purified capsular polysaccharides. One limitation of Polysaccharides vaccines is their inability to activate TH cells. They activate B cells in a thymus-independent type – 2(TI-2) manner, resulting in Ig M production but little class switching, no affinity maturation and little, if any, development of memory cells.
c. Synthetic Peptide Vaccines:
The development of synthetic peptides that might be useful as vaccines depends on the identification of immunogenic sites. Several methods have been used. The best known example is foot and mouth disease, where protection was achieved by immunizing animals with a linear sequence of 20 aminoacids. Synthetic peptide vaccines would have many advantages. Their antigens are precisely defined and free from unnecessary components which may be associated with side effects. They are stable and relatively cheap to manufacture. Furthermore, less quality assurance is required. Changes due to natural variation of the virus can be readily accommodated, which would be a great advantage for unstable viruses such as influenza. Synthetic peptides do not readily stimulate T cells. It was generally assumed that, because of their small size, peptides would behave like haptens and would therefore require coupling to a protein carrier which is recognized by T-cells. It is now known that synthetic peptides can be highly immunogenic in their free form provided they contain, in addition to the B cell epitope, T- cell epitopes recognized by T-helper cells. Such T-cell epitopes can be provided by carrier protein molecules, foreign antigens. or within the synthetic peptide molecule itself.
Synthetic peptides are not applicable to all viruses. This approach did not work in the case of polioviruses because the important antigenic sites were made up of 2 or more different viral capsid proteins so that it was in a concise 3-D conformation.
Advantages of defined viral antigens or peptides include:
1. Production and quality control simpler
2. No NA or other viral or external proteins, therefore less toxic.
3. Safer in cases where viruses are oncogenic or establish a persistent infection
4. Feasible even if virus cannot be cultivated.
Disadvantages:
1. May be less immunogenic than conventional inactivated whole-virus vaccines
2. Requires adjuvant
3. Requires primary course of injections followed by boosters
4. Fails to elicit CMI.
III. RECOMBINANT VACCINES:
a. Recombinant viral proteins
Virus proteins have been expressed in bacteria, yeast, mammalian cells, and viruses. E.Coli cells were first to be used for this purpose but the expressed proteins were not glycosylated, which was a major drawback since many of the immunogenic proteins of viruses such as the envelope glycoproteins, were glycosylated. Nevertheless, in many instances, it was demonstrated that the non-glycosylated protein backbone was just as immunogenic. Recombinant hepatitis B vaccine is the only recombinant vaccine licensed at present.
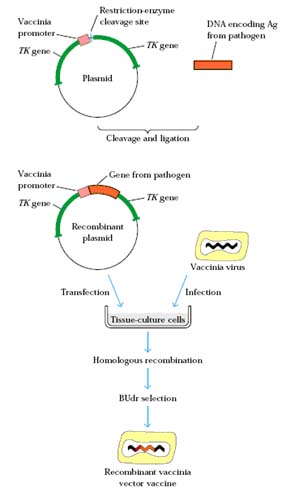
An alternative application of recombinant DNA technology is the production of hybrid virus vaccines. The best known example is vaccinia; the DNA sequence coding for the foreign gene is inserted into the plasmid vector along with a vaccinia virus promoter and vaccinia thymidine kinase sequences. The resultant recombination vector is then introduced into cells infected with vaccinia virus to generate a virus that expresses the foreign gene. The recombinant virus vaccine can then multiply in infected cells and produce the antigens of a wide range of viruses. The genes of several viruses can be inserted, so the potential exists for producing polyvalent live vaccines. HBsAg, rabies, HSV and other viruses have been expressed in vaccinia. Hybrid virus vaccines are stable and stimulate both cellular and humoral immunity. They are relatively cheap and simple to produce. Being live vaccines, smaller quantities are required for immunization. As yet, there are no accepted laboratory markers of attenuation or virulence of vaccinia virus for man. Alterations in the genome of vaccinia virus during the selection of recombinant may alter the virulence of the virus. The use of vaccinia also carries the risk of adverse reactions associated with the vaccine and the virus may spread to susceptible contacts. At present, efforts are being made to attenuate vaccinia virus further and the possibility of using other recombinant vectors is being explored, such as attenuated poliovirus and adenovirus.
b. Recombinant Vector Vaccines:
Vectored vaccines are now commercially available for Newcastle disease in poultry and for use in vaccinating wildlife against rabies. Additional vaccines are being tested for licensure. These vaccines seem to have all of the characteristics of an ideal vaccine. They are free of adverse side effects, stable, adaptable to mass vaccination, nonadjuvanted, and like the gene-deleted vaccines, allow for differentiation between a vaccinated and an infected animal. These vaccines are created by recombinant technology wherein the vector is deleted of one or more of its own genes, and into these sites, one or more protective genes from the pathogen are inserted into the genome of the vector. The vector is then administered as the vaccine, and the inserted gene products are produced by the vaccinator’s own body cells when infected by the vector. The vector may be severely attenuated so that it will not be shed from the vaccinae, or it may be hostrestricted so that it will not replicate itself within the tissues of the vaccinate. Early data collected on these vaccines indicate strong immunity, no side effects, and no shedding into the environment when distributed into the habitat of the species being immunized.
c. Subunit Vaccines:
Subunit vaccines are defined as those containing one or more pure or semi-pure antigens. In order to develop subunit vaccines, it is critical to identify the individual components out of a myriad of proteins and glycoproteins of the pathogen that are involved in inducing protection. Indeed, some proteins, if included in the vaccine, may be immunosuppressive, whereas in other cases immune responses to some proteins may actually enhance disease. Thus, it is critical to identify those proteins that are important for inducing protection and eliminate the others. Combining genomics with our understanding of pathogenesis, it is possible to identify specific proteins from most pathogens that are critical in inducing the immune responses. The potential advantages of using subunits as vaccines are the increased safety, less antigenic competition, since only a few components are included in the vaccine, ability to target the vaccines to the site where immunity is required, and the ability to differentiate vaccinated animals from infected animals (marker vaccines). One of the disadvantages of subunit vaccines is that they generally require strong adjuvants and these adjuvants often induce tissue reactions.
Secondly, duration of immunity is generally shorter than with live vaccines. In addition to using a whole protein as a vaccine, it is possible to identify individual epitopes within these protective proteins and develop peptide vaccines. The major disadvantage of peptide vaccines is that they often need to be linked to carriers to enhance their immunogenicity and, secondly, a pathogen can escape immune responses to a single epitope versus multiple epitope vaccines. To overcome some of these disadvantages, chimeric peptides can be made to broaden the immune response to different epitopes. Subunit vaccines are derived from recombinant organisms into which a foreign gene from a specific pathogen has been inserted. The recombinant organism is propagated and the protein encoded by the inserted gene is harvested, purified, and administered as a vaccine. These vaccines have not performed as well as had been originally anticipated because many of the proteins lacked the glycosylation that was essential for the antigen to be fully recognized and processed by the immune system of the vaccinate. Subunits may also be derived by purifying individual components of a whole cell culture.
d. Polynucleotide Vaccines:
The most recent development in vaccinology is immunization with polynucleotides. This technology has been referred to as genetic immunization or DNA immunization. The basis for this approach to immunization, is that cells can take-up plasmid DNA and express the genes within the transfected cells. Thus, the animal acts as a bioreactor to produce the vaccine. This makes the vaccine relatively inexpensive to produce. Some of the advantages of polynucleotide immunization is that it is extremely safe, induces a broad range of immune responses (cellular and humoral responses), long-lived immunity, and, most importantly, can induce immune responses in the presence of maternal antibodies. Most recently, it has also been used for immunizing fetuses. Thus, animals are born immune to the pathogens and at no time in the animal's life are they susceptible to these infectious agents. Although this is one of the most attractive developments in vaccinology, there is a great need to develop better delivery systems to improve the transfection efficiency in vivo.
DNA VACCINE:
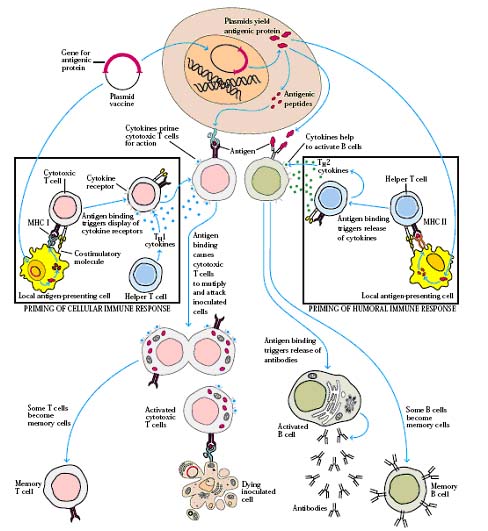
Recently, encouraging results were reported for DNA vaccines whereby DNA coding for the foreign antigen is directly injected into the animal so that the foreign antigen is directly produced by the host cells. In theory these vaccines would be extremely safe and devoid of side effects since the foreign antigens would be directly produced by the host animal. In addition, DNA is relatively inexpensive and easier to produce than conventional vaccines and thus this technology may one day increase the availability of vaccines to developing countries. Moreover, the time for development is relatively short which may enable timely immunization against emerging infectious diseases. In addition, DNA vaccines can theoretically result in more long-term production of an antigenic protein when introduced into a relatively nondividing tissue, such as muscle. Indeed some observers have already dubbed the new technology the "third revolution" in vaccine development—on par with Pasteur's ground-breaking work with whole organisms and the development of subunit vaccines. The first clinical trials using injections of DNA to stimulate an immune response against a foreign protein began for HIV in 1995. Four other clinical trials using DNA vaccines against influenza, herpes simplex virus, T-cell lymphoma, and an additional trial for HIV were started in 1996. The technique that is being tested in humans involves the direct injection of plasmids - loops of DNA that contain genes for proteins produced by the organism being targeted for immunity. Once injected into the host's muscle tissue, the DNA is taken up by host cells, which then start expressing the foreign protein. The protein serves as an antigen that stimulates an immune responses and protective immunological memory. Enthusiasm for DNA vaccination in humans is tempered by the fact that delivery of the DNA to cells is still not optimal, particularly in larger animals. Another concern is the possibility, which exists with all gene therapy, that the vaccine's DNA will be integrated into host chromosomes and will turn on oncogenes or turn off tumor suppressor genes. Another potential downside is that extended immunostimulation by the foreign antigen could in theory provoke chronic inflammation or autoantibody production.
IV. FUTURE VACCINES:
a. Multivalent Subunit Vaccines:
One of the limitations with synthetic peptide vaccines and recombinant protein vaccines is that these vaccines tend to be poorly immunogenic; in addition, they tend to induce a humoral antibody response but are less likely to induce a cell-mediated response. To make these vaccines active, it should contain both immunodominant B-cell and T-cell epitopes and they must be delivered intracellularly so that the peptides can be processed and presented together with class I and class II molecules. Multivalent subunit vaccines satisfy all these needs. They are named to so, because they contain epitopes of different organism in same vaccine. There are four different components can act as multivalent subunit vaccines namely Solid Matrix-antibody-antigen complex, Micelles, Liposomes and Immunostimulating complexes.
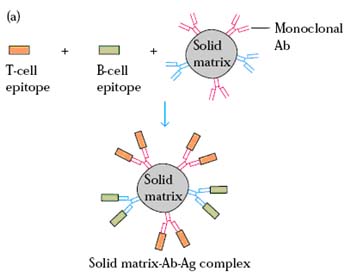
Solid Matrix Antibody Antigen (SMAA) Complex:
It is prepared by attaching monoclonal antibodies to particulate solid matrices and then saturating the antibody with the desired antigens. The resulting SMAA complex is then used as vaccines. These multivalent complexes have been shown to induce vigorous humoral and cell-mediated responses. Their particulate nature contributes to their increased immunogenicity by facilitating phagocytosis by phagocytic cells.
Micelles:
They are formed by mixing proteins (antigens) in detergent and then removing the detergent. The individual proteins will orient themselves with the hydrophilic residues oriented toward the aqueous environment and the hydrophobic residues at the centre so as to exclude their interaction with the aqueous environment. Then they are used as vaccines.
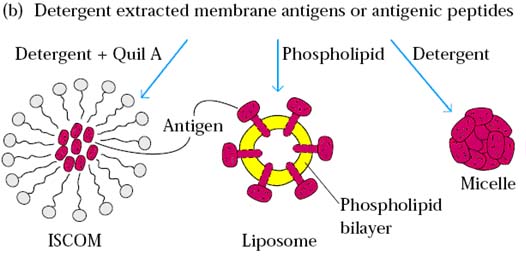
Liposomes:
Liposomes containing protein antigens are prepared by mixing the proteins (antigens) with a suspension of phospholipids under conditions that form vesicles bounded by a bilayer. The proteins are incorporated into the bilayer with the hydrophilic residues exposed. These liposomes were then used as vaccines.
Immunostimulating complexes (ISCOMS)
It is an alternative vaccine vehicle. The antigen is presented in an accessible, multimeric, physically well defined complex. Composed of adjuvant (Quil A) and antigen held in a cage like structure. Adjuvant is held to the antigen by lipids. It can stimulate CMI. Its mean diameter is 35nm.
In the most successful procedure, a mixture of the plant glycoside saponin, cholesterol and phosphatidylcholine provides a vehicle for presentation of several copies of the protein on a cage-like structure. Such a multimeric presentation mimics the natural situation of antigens on microorganisms. These Immunostimulating complexes have activities equivalent to those of the virus particles from which the proteins are derived, thus holding out great promise for the presentation of genetically engineered proteins.
Similar considerations apply to the presentation of peptides. It has been shown that by building the peptide into a framework of lysine residues so that 8 copies instead of 1 copy are present, the immune response induced was of a much greater magnitude. A novel approach involves the presentation of the peptide in a polymeric form combined with T cell epitopes. The sequence coding for the foot and mouth disease virus peptide was expressed as part of a fusion protein with the gene coding for the Hepatitis B core protein. The hybrid protein, which forms spherical particles 22nm in diameter, elicited levels of neutralizing antibodies against foot and mouth disease virus that were at least a hundred times greater than those produced by the monomeric peptide.
Action of ISCOM:
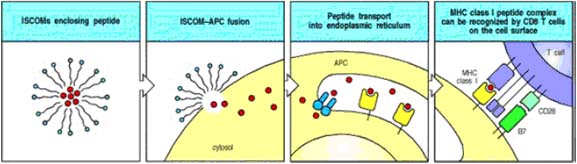
ISCOMs (immune stimulatory complexes) are lipid micelles that will fuse with cell membranes. Peptides trapped in ISCOMs can be delivered to the cytosol of an antigenpresenting cell (APC), allowing the peptide to be transported into the endoplasmic reticulum, where it can be bound by newly synthesized MHC class I molecules and hence transported to the cell surface as peptide:MHC class I complexes. This is a possible means for delivering vaccine peptides to activate CD8 cytotoxic T cells. ISCOMs can also be used to deliver proteins to the cytosol of other types of cell, where they can be processed and presented as though they were a protein produced by the cell.
b. Anti-idiotype antibodies:
The ability of anti-idiotype antibodies to mimic foreign antigens has led to their development as vaccines to induce immunity against viruses, bacteria and protozoa in experimental animals. Anti-idiotypes have many potential uses as viral vaccines, particularly when the antigen is difficult to grow or hazardous. They have been used to induce immunity against a wide range of viruses, including HBV, rabies, Newcastle disease virus and FeLV, reoviruses and polioviruses.
c. Vaccine production in plants:
As a new direction in the development of affordable new vaccines, transgenic plants have been developed that express surface proteins of viruses that are pathogenic to animals or humans. For example, Agricultural Genetics in England has genetically engineered the cowpea mosaic virus to include a surface antigen from the foot-and-mouth disease virus; this virus affects life stock. This genetically engineered virus was used to infect its natural host, black-eyed pea, and the introduced gene from the foot-and-mouth disease virus was expressed handsomely in the plant. The cowpea mosaic virus eventually kills the plant, and therefore the plant needs to be sacrificed a few weeks after infection. One leaf from the infected pea plant produces enough surface antigen to serve as vaccine for 200 doses.