Immune system
The immune system is a network of cells, tissues, and organs that work together to defend the body against attacks by “foreign” invaders. These are primarily microbes—tiny organisms such as bacteria, parasites, and fungi that can cause infections. Viruses also cause infections, but are too primitive to be classified as living organisms. The human body provides an ideal environment for many microbes. It is the immune system’s job to keep them out or, failing that, to seek out and destroy them.
When the immune system hits the wrong target, however, it can unleash a torrent of disorders, including allergic diseases, arthritis, and a form of diabetes. If the immune system is crippled, other kinds of diseases result.
The immune system is amazingly complex. It can recognize and remember millions of different enemies, and it can produce secretions (release of fluids) and cells to match up with and wipe out nearly all of them.
Structure of Immune system:


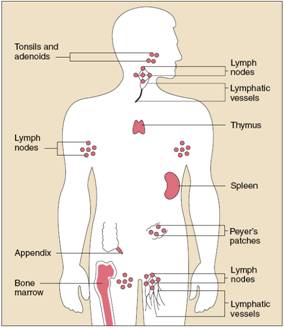
The organs of the immune system are positioned throughout the body. They are called lymphoid organs because they are home to lymphocytes, small white bloodcells that are the key players in the immune system.
Bone marrow, the soft tissue in the hollow
center of bones, is the ultimate source of all blood cells, including
lymphocytes. The thymus is a lymphoid organ that lies behind the breastbone.
Lymphocytes known as T lymphocytes or T cells (“T” stands for “thymus”) mature
in the thymus and then migrate to other tissues. B lymphocytes, also known as B
cells, become activated and mature into plasma cells, which make and release
antibodies.
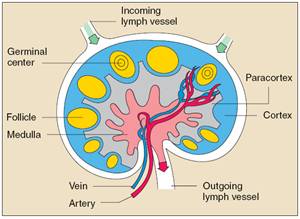
Lymph nodes, which are located in many parts of the body, are lymphoid tissues that contain numerous specialized structures.
Lymphocytes can travel throughout the body using the blood vessels. The cells can also travel through a system of lymphatic vessels that closely parallels the body’s veins and arteries.
Cells and fluids are exchanged between blood and lymphatic vessels, enabling the lymphatic system to monitor the body for invading microbes. The lymphatic vessels carry lymph, a clear fluid that bathes the body’s tissues.
Small, bean-shaped lymph nodes are laced along the lymphatic vessels, with clusters in the neck, armpits, abdomen, and groin. Each lymph node contains specialized compartments where immune cells congregate, and where they can encounter antigens.
Immune cells, microbes, and foreign antigens enter the lymph nodes via incoming lymphatic vessels or the lymph nodes’ tiny blood vessels. All lymphocytes exit lymph nodes through outgoing lymphatic vessels. Once in the bloodstream, lymphocytes are transported to tissues throughout the body. They patrol everywhere for foreign antigens, then gradually drift back into the lymphatic system to begin the cycle all over again.
The spleen is a flattened organ at the upper left of the abdomen. Like the lymph nodes, the spleen contains specialized compartments where immune cells gather and work. The spleen serves as a meeting ground where immune defenses confront antigens.
Other clumps of lymphoid tissue are found in many parts of the body, especially in the linings of the digestive tract, airways, and lungs—territories that serve as gateways to the body. These tissues include the tonsils, adenoids, and appendix.
Immune cells:
The immune system stockpiles a huge arsenal of cells, not only lymphocytes but also cell-devouring phagocytes and their relatives. Some immune cells take on all intruders, whereas others are trained on highly specific targets. To work effectively, most immune cells need the cooperation of their comrades. Sometimes immune cells communicate by direct physical contact, and sometimes they communicate releasing chemical messengers.
All immune cells begin as immature stem cells in the bone marrow. They respond to different cytokines and other chemical signals to grow into specific immune cell types, such as T cells, B cells, or phagocytes. Because stem cells have not yet committed to a particular future, their use presents an interesting possibility for treating some immune system disorders
B-cell
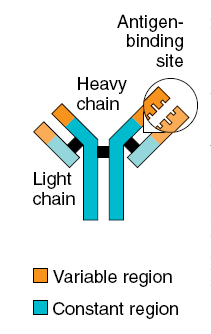
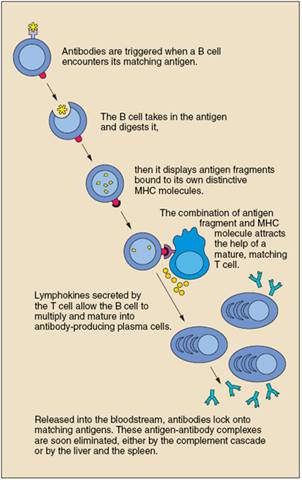
B cells work chiefly by secreting substances called antibodies into the body’s fluids. Antibodies ambush foreign antigens circulating in the bloodstream. They are powerless, however, to penetrate cells. Each B cell is programmed to make one specific antibody. For example, one B cell will make an antibody that blocks a virus that causes the common cold, while another produces an antibody that attacks a bacterium that causes pneumonia. When a B cell encounters the kind of antigen that triggers it to become active, it gives rise to many large cells known as plasma cells, which produce antibodies.
T-cells:
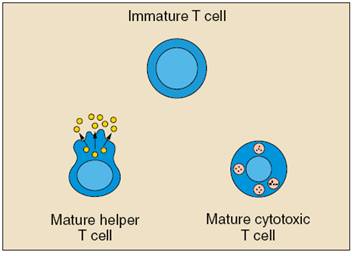
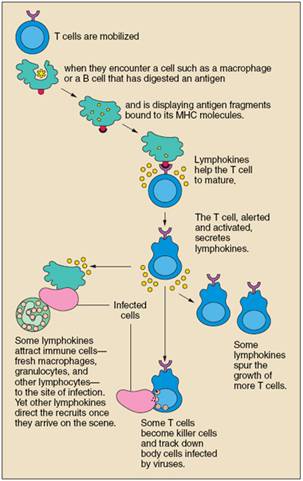
Unlike B cells, T cells do not recognize free-floating antigens. Rather, their surfaces contain specialized antibody-like receptors that see fragments of antigens on the surfaces of infected or cancerous cells. T cells contribute to immune defenses in two major ways: Some direct and regulate immune responses, whereas others directly attack infected or cancerous cells.
Helper T cells, or Th cells, coordinate immune responses by communicating with other cells. Some stimulate nearby B cells to produce antibodies, others call in microbe-gobbling cells called phagocytes, and still others activate other T cells.
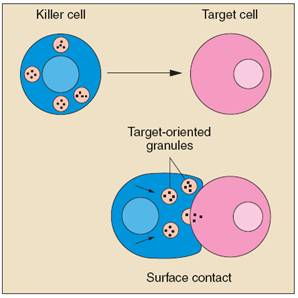
Cytotoxic T lymphocytes (CTLs)—also called killer T cells—perform a different function. These cells directly attack other cells carrying certain foreign or abnormal molecules on their surfaces. CTLs are especially useful for attacking viruses because viruses often hide from other parts of the immune system while they grow inside infected cells. CTLs recognize small fragments of these viruses peeking out from the cell membrane and launch an attack to kill the infected cell.
In most cases, T cells only recognize an antigen if it is carried on the surface of a cell by one of the body’s own major histocompatibility complex, or MHC, molecules. MHC molecules are proteins recognized by T cells when they distinguish between self and nonself. A self-MHC molecule provides a recognizable scaffolding to present a foreign antigen to the T cell. In humans, MHC antigens are called human leukocyte antigens, or HLA.
Although MHC molecules are required for T cell responses against foreign invaders, they also create problems during organ transplantations. Virtually every cell in the body is covered with MHC proteins, but each person has a different set of these proteins on his or her cells. If a T cell recognizes a nonself-MHC molecule on another cell, it will destroy the cell. Therefore, doctors must match organ recipients with donors who have the closest MHC makeup. Otherwise the recipient’s T cells will likely attack the transplanted organ, leading to graft rejection.
NK cells:
Natural killer (NK) cells are another kind of lethal white cell, or lymphocyte. Like CTLs, NK cells are armed with granules filled with potent chemicals. But CTLs look for antigen fragments bound to self-MHC molecules, whereas NK cells recognize cells lacking self-MHC molecules. Thus, NK cells have the potential to attack many types of foreign cells.
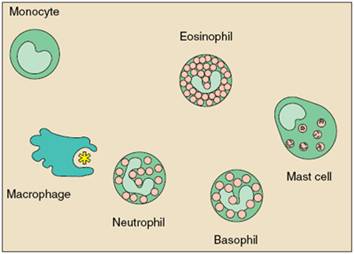
Phagocytes and Their Relatives:
Phagocytes are large white cells that can swallow and digest microbes and other foreign particles. Monocytes are phagocytes that circulate in the blood. When monocytes migrate into tissues, they develop into macrophages. Specialized types of macrophages can be found in many organs, including the lungs, kidneys, brain, and liver.
Macrophages play many roles. As scavengers, they rid the body of worn-out cells and other debris. They display bits of foreign antigen in a way that draws the attention of matching lymphocytes and, in that respect, resemble dendritic cells. And they churn out an amazing variety of powerful chemical signals, known as monokines, which are vital to the immune response.
Granulocytes are another kind of immune cell. They contain granules filled with potent chemicals, which allow the granulocytes to destroy microorganisms. Some of these chemicals, such as histamine, also contribute to inflammation and allergy.
One type of granulocyte, the neutrophil, is also a phagocyte. Neutrophils use their prepackaged chemicals to break down the microbes they ingest. Eosinophils and basophils are granulocytes that “degranulate” by spraying their chemicals onto harmful cells or microbes nearby.
Mast cells function much like basophils, except they are not blood cells. Rather, they are found in the lungs, skin, tongue, and linings of the nose and intestinal tract, where they contribute to the symptoms of allergy.
Related structures, called blood platelets, are cell fragments. Platelets also contain granules. In addition to promoting blood clotting and wound repair, platelets activate some immune defenses.
Dendritic cells are found in the parts of lymphoid organs where T cells also exist. Like macrophages, dendritic cells in lymphoid tissues display antigens to T cells and help stimulate T cells during an immune response. They are called dendritic cells because they have branchlike extensions that can interlace to form a network.
Immune Response
Infections are the most common cause of human disease. They range from the common cold to debilitating conditions like chronic hepatitis to life-threatening diseases such as AIDS. Disease-causing microbes (pathogens) attempting to get into the body must first move past the body’s external armor, usually the skin or cells lining the body’s internal passageways.
The skin provides an imposing barrier to invading microbes. It is generally penetrable only through cuts or tiny abrasions. The digestive and respiratory tracts—both portals of entry for a number of microbes—also have their own levels of protection. Microbes entering the nose often cause the nasal surfaces to secrete more protective mucus, and attempts to enter the nose or lungs can trigger a sneeze or cough reflex to force microbial invaders out of the respiratory passageways. The stomach contains a strong acid that destroys many pathogens that are swallowed with food.
If microbes survive the body’s front-line defenses, they still have to find a way through the walls of the digestive, respiratory, or urogenital passageways to the underlying cells. These passageways are lined with tightly packed epithelial cells covered in a layer of mucus, effectively blocking the transport of many pathogens into deeper cell layers.
Mucosal surfaces also secrete a special class of antibody called IgA, which in many cases is the first type of antibody to encounter an invading microbe. Underneath the epithelial layer a variety of immune cells, including macrophages, B cells, and T cells, lie in wait for any microbe that might bypass the barriers at the surface.
Next, invaders must escape a series of general defenses of the innate immune system, which are ready to attack without regard for specific antigen markers. These include patrolling phagocytes, natural killer T cells, and complement.
Microbes cross the general barriers then confront specific weapons of the adaptive immune system tailored just for them. These specific weapons, which include both antibodies and T cells, are equipped with singular receptor structures that allow them to recognize and interact with their designated targets.
Bacteria, Viruses, and Parasites
The most common disease-causing microbes are bacteria, viruses, and parasites. Each uses a different tactic to infect a person, and, therefore, each is thwarted by different components of the immune system.
Most bacteria live in the spaces between cells and are readily attacked by antibodies. When antibodies attach to a bacterium, they send signals to complement proteins and phagocytic cells to destroy the bound microbes. Some bacteria are eaten directly by phagocytes, which signal to certain T cells to join the attack.
All viruses, plus a few types of bacteria and parasites, must enter cells of the body to survive, requiring a different kind of immune defense. Infected cells use their major histocompatibility complex molecules to put pieces of the invading microbes on their surfaces, flagging down cytotoxic T lymphocytes to destroy the infected cells. Antibodies also can assist in the immune response by attaching to and clearing viruses before they have a chance to enter cells.
Parasites live either inside or outside cells. Intracellular parasites such as the organism that causes malaria can trigger T cell responses. Extracellular parasites are often much larger than bacteria or viruses and require a much broader immune attack. Parasitic infections often trigger an inflammatory response in which eosinophils, basophils, and other specialized granule-containing cells rush to the scene and release their stores of toxic chemicals in an attempt to destroy the invaders. Antibodies also play a role in this attack, attracting the granule-filled cells to the site of infection.
Immunity and Nutrition:
Malnutrition has an adverse impact on cell-mediated, secretory and humoral immunity, as well as on non-specific host defences. Although most clinical observations concerning this problem have been made in patients with generalized malnutrition, deficiencies or excess of single nutrients can also cause acquired functional derangements. Acquired anergy can generally be reversed when malnutrition is corrected. Further, secondary nutritional derangements tend to be less severe than primary congenital defects of an immune or non-specific defence component. Unlike the relative resistance to infection seen in patients with uncomplicated starvation, cachexia associated with severe disease, malignancy, or trauma is typically accompanied by superimposed infections, especially due to opportunistic microorganisms that are normally of low pathogenicity. The hypermetabolism of most severe diseases is accompanied by complex biochemical responses, increased energy generation, and an inability to conserve amino acid stores. Secondary derangements in the function of defensive mechanisms and immune responses may then emerge rapidly. Further, loss of body nutrients during primary infection increases the susceptibility of a patient to secondary infections. Chronically malnourished patients often exhibit an infection or infestation as a coexisting problem.
Nutritional effects on immune mechanisms
Although generalized malnutrition can affect all aspects of host immunity, the impact if greatest on T cell functions and cell-mediated immunity and smallest on B cell functions and humoral immunity. Effects on secretory immunity fall in between.
Slight leukopenia, together with relative lymphocytosis was found in patients suffering from eating disorders. In fact, plasma concentrations of IgM, IgG, IgA, IgD, and IgE may all be greater than normal in malnourished infants, and blocking antibodies and antibodies against food antigens may increase. The humoral immunity, judged by serum IgA concentration, was different in adolescent females suffering from obesity or anorexia nervosa; obese patients showed the highest values.
Apparently paradoxical increases in plasma Ig values during malnutrition have not been explained with certainty, but may involve the presence of infectious or parasitic diseases. Children of developing countries tend to experience a continuing heavy exposure to multiple antigens. Vitamin A supplementation results in an increased IgG response to the wild measles virus. During severe generalized malnutrition, lymphoid tissue atrophy develops primarily in the T cell areas and circulating T cell numbers decline. Loss of delayed dermal hypersensitivity to previously encountered antigens is typical and de novo dermal sensitization responses are impaired. In vitro responsiveness to mitogens and antigens is impaired in T cells from patients with generalized protein-energy malnutrition. Host-versus-graft reactions are delayed.
Protein undernutrition has been shown to suppress significantly the secretion of lysozyme into tears. Lysozyme levels in tears were significantly lower (50% less) in moderately malnourished grade II children than in normal children. The synthesis and secretion into tears of other locally produced proteins, slgA and amylase were also impaired. Decreased activity of lysozyme has also been observed in leukocytes of children with protein-calorie malnutrition (PCM). Reduced concentrations of slgA and, to lesser extent, lysozyme, are potentially important in the defence of mucosal surfaces. The lower levels differ significantly from those serum proteins, such as total protein, IgG, aminopeptidase, and albumin, levels of which in tears were not influenced by nutritional status. This indicates that the reduced levels of slgA, amylase and lysozyme were not due to reduced volume of secretion in malnourished children, as has been suggested by experimental studies on saliva in malnourished rats. It appears that the mechanism involves impairment in the local synthesis and/or secretion of these proteins. These observations have been confirmed by studying parotid saliva during the renutrition of children with kwashiorkor or marasmic malnutrition. Saliva flow rate (millimetre per minute) increased about 50% during a 4-week renutrition with a high protein diet. Therefore, the non-specific immunity due to the washing effects of secretions was significantly increased. Decreased total salivary IgA levels in children with acquired immune deficiency syndrome (AIDS), with a compromised nutritional status were reported.
Imume system behaviour under malnutrition
The development, maintenance and optimal functioning of the immune system depend on balanced and adequate nutrition. Both deficiency and excess of a number of nutrients adversely affect the number and activity of immune cells. In this sense, many metabolic pathways require specific nutrients as cofactors. The antioxidative defence mechanisms employed by the body are also heavily dependent on the nutritional intake of the individual and involve a variety of vitamins (e.g. vitamins C and E), trace elements (e.g. zinc and selenium) and amino acids (e.g. cysteine). Multiple- rather than single-nutrient deficiencies are often the causes for a compromised immune system and an increased risk of infection, as observed in patients with protein-energy malnutrition (PEM).
In malnourished subjects anatomical changes involve atrophy of primary as well as secondary immune organs such as the thymus and spleen, respectively. Malnutrition impairs mainly cell-mediated immunity, which is altered at an early stage in the development of undernutrition. Nevertheless, some alterations in phagocytosis and the complement system have also been described in PEM. B cell function has so far not been shown to be affected by PEM to the same extent as that of T lymphocytes. In addition, the production of several cytokines, such as interleukin-1 (IL-1), interleukin-2 (IL-2), and interferon-y (IFN-y), is decreased. Moreover, malnutrition alters the ability of T lymphocytes to respond appropriately to cytokines. There is little work on the effect of malnutrition on the integrity of physical barriers, quality of mucus, or several other innate immune defences. For example, lysozyme concentrations are decreased, largely as the result of reduced production in monocytes and neutrophils and an increased excretion in urine.
Effects of micronutrients on the immune system
Several trace elements and vitamins have an essential role in key metabolic pathways of immune cells functions. Isolated deficiencies of micronutrients are rare with the exception of zinc, iron, and vitamin A. Observations in laboratory animals and findings in the rare patient with a single nutrient deficiency have confirmed the crucial role of several vitamins and trace elements in immunocompetence. Alterations in immune responses occur early in the course of reduction in micronutrient intake, and the extent of immunological impairment depends on the type of nutrient involved, its interactions with other essential nutrients, the severity of the deficiency, the presence of concomitant infection, and the age of the subject. Thus, tests of immunocompetence are useful in titration of physiological needs and in assessment of safe lower and upper limits of micronutrient intakes.
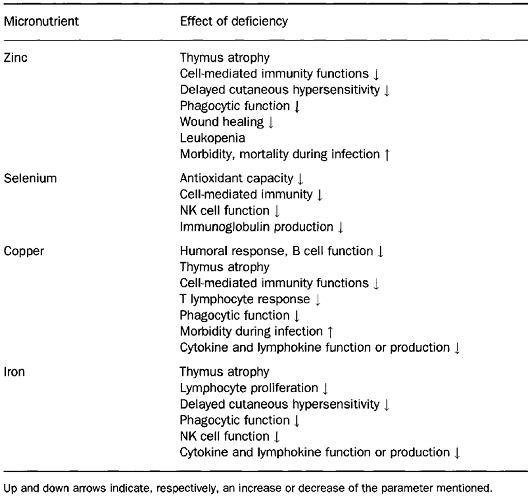
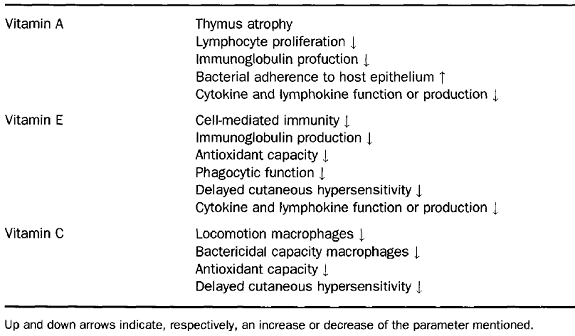
Many studies have pointed out that micronutrients such as zinc, selenium, iron, copper, carotene, vitamins A, C, and E and folic acid can influence several components of the nonspecific immunity.
Zinc
The essentiality of zinc for humans was already first documented in the 1960s. In human subjects body growth and development are strictly dependent on zinc. The nervous, reproductive and immune systems are particularly influenced by zinc deficiency, as well as by increased levels of zinc.
More than 300 metalloenzymes have been identified as being zinc (Zn) dependent. Many of these are critical for cellular metabolic pathways, including those that mediate phagocyte and lymphocyte functions. Zinc deficiency is associated with lymphoid atrophy, decreased delayed-type hypersensitivity (DTH) cutaneous responses, delayed homograft rejection, and a lower thymulin (thymic hormone) activity. It is not surprising then that zinc deficiency results in profound immunodeficiency. The salient changes observed are in
(a) phagocytes: reduced ingestion of microorganisms, impaired chemotactic migration, decreased activity of reduced oxidase, which is a cofactor for phospholipases A2 and C and instability of cell membranes possibly due to oxidation of arachidonic acid by iron complexes;
(b) cell-mediated immunity: imbalance between T helper type 1 (Thl) and Th2 functions, reduced lymphocyte proliferation response, decreased CD4 : CDS cell ratio and helper T cell function, impaired natural killer (NK) cell function, reduced thymulin activity (a thymic hormone); and
(c) humoral immunity: decreased antibody production after challenge with T cell dependent antigens and alloantigens.
A slightly excessive intake of certain nutrients such as zinc may be associated with enhanced immune responses.
The moderate deficiencies in zinc noted in sickle cell anaemia, renal disease, chronic gastrointestinal disorders, acrodermatitis enteropathica, virus-associated immunodeficiency, diarrhoeas, and in elderly persons can greatly alter host defence systems, leading to increases in opportunistic infections and mortality rates. Conversely, short periods of Zn supplementation substantially improve immune defense in individuals with these diseases.
Selenium
The trace mineral selenium is an essential nutrient of fundamental importance to human biology. As selenocysteine, the 21st amino acid, selenium (Se) is a component of selenoproteins, some of which have important enzymatic functions. In the active site selenium functions as a redox centre; the best-known example of this redox function is the reduction of hydrogen peroxide and damaging lipid and phospholipid hydroperoxides to harmless products (water and alcohols) by the family of selenium-dependent glutathione peroxidases. This function helps to maintain membrane integrity, protects prostacyclin production, and reduces the likelihood of propagation of further oxidative damage to biomolecules such as lipids, lipoproteins, and DNA with the associated increased risk of conditions such as atherosclerosis and cancer.
Selenium has additional important health effects particularly in relation to the immune response and viral disease. This trace mineral is normally found in significant amounts in immune tissues such as liver, spleen, and lymph nodes. Both cellmediated immunity and B cell function can be impaired in selenium deficiency. By way of contrast, supplementation with selenium has marked immunostimulating effects, including an enhancement of proliferation of activated cytotoxic T cells and an increased NK cell activity. These effects have been related to the ability of selenium to enhance interleukin-2 receptor (IL-2R) expression on the surface of activated lymphocytes and NK cells.
Copper
Copper is known to play an important role in the development and maintenance of the immune system but its exact mechanism of action is not yet known. The role of copper includes both B cell and T cell related deficiencies. Impaired antibody formation, inflammatory response, phagocytic killing power, and lymphocyte stimulation responses, as well as thymic atrophy, have been well documented.
Some of the previous research showed that IL-2 is reduced in copper deficiency and is likely the mechanism for T cell proliferation reduction. Similarly, the number of neutrophils in human peripheral blood and their ability to generate superoxide anion and kill ingested microorganisms is reduced in both overt and marginal copper deficiency. In addition, infections decrease both serum copper and zinc aggravating the situation and further impairing the defence system. Neutrophillike HL-60 cells accumulate copper as they differentiate into a more mature cell population and this accumulation is not reflected by increases in Cu/Zn superoxide dismutase or cytochrome-c oxidase activities. The identity of copper-binding proteins in neutrophil-like HL-60 may be useful in learning new functions of copper.
Iron
Iron deficiency is the most widespread nutrient deficiency in the world today. There is a large body of evidence accumulated from animal and human studies to indicate that iron deficiency states are associated with alterations in cellular function, growth, motor development, behaviour and cognitive function. No controversy exists about the deleterious effects of iron deficiency on immune responses; almost all published studies indicate that individuals with iron deficiency show impairment of cell-mediated immunity (DTH responses, T lymphocyte proliferation response to mitogens and antigens, production of cytokines such as IL-2 and IFN-y). In addition, phagocyte microbicidal function, NK activity and mucosal immunity are impaired. Apparently B cell and antibody formation are not affected. These alterations may well be linked to changes in the activity of iron-dependent enzymes such as myeloperoxidase and ribonucleotide reductase. In addition, physical changes in the mucosal epithelia may also be important. A more recent study indicates that iron presence can help monocytes to suppress Mycobacterium tuberculosis growth.
Iron-mediated growth suppression was correlated with selective suppression of tumour necrosis factor-a (TNF-a) release from infected monocytes and decreased monocyte sensitivity to exogenously added TNF. Transferrin is found not only in blood but also in all body fluids and is the normal mechanism for withholding iron from the infectious agent, as is lactoferrin. Conalbumin and lactoferrin have stronger iron binding properties than do most bacterial siderophores and are normally highly unsaturated. When molecules of lactoferrin become 40% saturated with iron, they are assimilated by macrophages that have been attracted to the site of infection and much of the iron is incorporated into ferritin.
Ferritin functions as an iron withholding rather than an iron-transport agent. In an iron-deficient host with reduced immune function, lack of available iron for agent replication is protective. When individuals whose resistance to infection is compromised by iron deficiency are given parenteral iron or large doses of oral iron, a disastrous exacerbation of the infection and death may occur. This happens because the agent is supplied with iron for replication before the host immune system has had time to recover. However, in field studies, supplementation of poorly nourished adults with physiological amounts of up to l00mg Fe/day and proportionately less for children, consistently results in decreased morbidity from infectious diseases.
Antioxidant vitamins
The oxidant/antioxidant balance is an important determinant of immune cell function, including maintaining integrity and functionality of membrane lipids, cellular proteins, nucleic acids, and for control of signal transduction and gene expression on immune cells. Thus, optimal function of the host defence system depends upon an adequate supply of antioxidant vitamins and, on the other hand, impaired host defence activity can act as a very early and sensitive marker of marginal deficiency of antioxidant vitamins. In humans, dietary supplementation with ascorbic acid, tocopherols and vitamin A has been shown to enhance a number of aspects of immunity and resistance to disease.
Vitamin A
Atrophy of lymphoid organs, including spleen, thymus, and lymph nodes, has been reported in vitamin A-deficient animals. However, some of these effects may be caused by loss of appetite and decreased food intake. Changes in spleen cell number are observed in the early stages of vitamin A deficiency and might be a more sensitive indicator of vitamin A deficiency, which can also affect the function of different cells of the immune system. In addition, vitamin A deficiency reduces NK activity, and is associated with a lower production of interferon.
Vitamin A is essential for the maintenance of epidermal and mucosal integrity, thus low plasma vitamin A concentrations have deleterious effects on membrane integrity and mucosal function. It is not surprising that impaired antibody response to viral and parasitic antigens has also been pointed out in vitamin A deficiency in animals. It is well-known that impaired intestinal immunoglobulin A (IgA) production is attributed to impairment of gut-associated immune response. As vitamin A deficiency has been associated with an increased morbidity and mortality from infectious diseases (diarrhoea and respiratory infections), several investigators have attempted to improve the immune response and, thus, resistance to infection by vitamin A supplementation in vitamin A-deficient subjects.
Vitamin E
Vitamin E is the major peroxidation chain-breaking antioxidant in membranes. Membrane phospholipids of immunocompetent cells have a high content of polyunsaturated fatty acids and are prime targets for free radical reactions. Release of reactive oxygen species by phagocytes on encountering pathogens and rapid lymphocyte proliferation following antigenic stimulation expose the immune cells to high levels of oxidative stress. Thus, it is not surprising that cells of the immune system have higher vitamin E content than other cells of the body. Both deficiency and supplementation of vitamin E have been shown to alter the immune response and resistance against infection. The influence of vitamin E on the immune function has been shown to affect different aspects of immune functions including T cell response, antibody production, NK cell activity, phagocytic activity, and the production of immunoregulatory molecules. In humans, primary deficiency of vitamin E rarely occurs, whereas secondary deficiency is observed as a consequence of certain diseases such as primary cirrhosis, intestinal malabsorption disorders and several viral hepatitis and human immunodeficiency virus (HIV)-l infection. In addition, vitamin E deficiency is associated with increased infectious diseases and the incidence of tumours. In contrast, vitamin E is one of the few nutrients for which supplementation at higher than established dietary requirements has been shown to enhance immune response and resistance to disease and this seems to be especially important in the aged. The beneficial effects of supplementation on the host immune system include enhanced humoral and cell-mediated immunity and increased efficacy of phagocytosis in humans.
Vitamin C
Vitamin C appears to affect most aspects of the immune system. It is found in high concentrations in leukocytes, it is rapidly utilized during infection, and reduced plasma levels are often associated with reduced immune function. However, the belief that high intakes of vitamin C will prevent the onset of common cold has not been scientifically substantiated. A decrease in the duration of cold episodes and the severity of symptoms seem more plausible, although the benefits that have been observed in different studies show a large variation. The explanation for this amelioration of symptoms could be derived from the decrease in the inflammatory effects due to the reaction of vitamin C with the phagocyte derived oxidants released extracellularly during infection episodes, thus being neutralized. In a placebo-controlled, double-blind intervention study, responses to DTH skin tests were significantly reduced in healthy men submitted to a vitamin C-deficient diet for 60 days. This study showed that moderate vitamin deficiency reduces cellmediated immunity. On the other hand, supplementation has been shown to increase neutrophil motility and mitogen-stimulated lymphocyte proliferation in young males as well as decrease the incidence of post-race infections in marathon runners.
Vitamin C is one of the food components with higher antioxidant properties as demonstrated, for instance, on the protection of lipids in plasma and low-density lipoproteins (LDL) against detectable peroxidative damage. Vitamin C also serves as an electron donor to vitamin E radicals generated in the cell membrane during oxidative stress. Some effects of vitamin C, such as the decrease in DNA damage by reactive oxygen species after supplementation, still remain to be established in humans. However, their function as antioxidant may be associated to atherosclerosis and cancer prevention. The effect of simultaneous supplementation with several vitamins over the immune system has been addressed many times. A significant decrease in the number of sick days and in the use of antibiotics, as well as an increased antibody response to the flu vaccine, in a group of healthy elderly subjects who supplemented their diets with a multivitamin supplement that contained 100% of the recommended daily allowance (RDA) of most vitamins and moderately higher amounts of antioxidant vitamin C (80 mg/day), vitamin E (44 mg/day), and (3-carotene (I6mg/day) was reported. The importance of maintaining adequate amounts of antioxidants throughout life, is based on a potential long-term effect of prevention of the accumulative damage caused by reactive oxygen species being made manifest in later years.
Dietary guidelines for disease prevention:
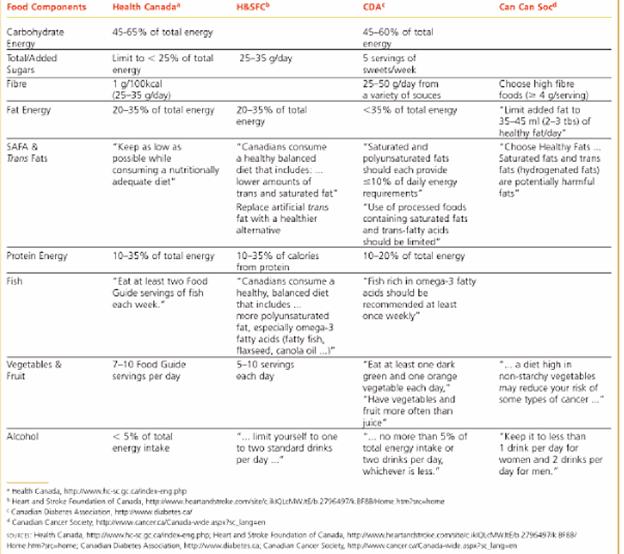
The choice of diet can influence long-term health prospects more than any other action one might take during their lifetime. People who consume the moderate, adequate, balanced, calorie-controlled diet recommended by ICMR may enjoy a longer, healthier life than those who do not. Following Table provides guidelines for disease prevention.
Reduce Saturated fat and Trans fat intake:
Primary among the recommendations is to choose unsaturated fats in place of saturated fat and trans fat. To meet this goal requires people to limit such foods as high-fat milk and dairy products, stick margarine, high-fat; baked goods and convenience foods, foods with high-fat creams and gravies, commercially fried foods, fat-marbled meat cuts, sausages, and fatty ground beef.
Conversely, foods such as nuts, olive oil, canola oil, and liquid margarines are high in polyunsaturated fats that supply that essential fatty acids and vitamin E. Because they are high in calories, use them only within your daily energy budget. Regular meals of fish, particularly fatty fish such as salmon, help balance intakes of omega-6 and omega-3 fatty acids.
Include Fruit, Vegetables and Whole grains:
Five or more vegetable and fruit food guide servings per day are recommended for those four years and up.
Eat at least one green and one orange vegetable per day and have vegetables and fruit more often than juice.
Every legitimate source of dietary advice urges people to include a variety of fruit and vegetables in the diet, not just for nutrients but also for the phytochemicals that combine synergistically to promote health. The grain foods consumed daily will supply nutrients, fibre and nonnutrients associated with good health.
Go for Variety:
Eat foods high in potassium (fruit and vegetables), high in calcium and magnesium (milk products and appropriate substitutes), low in fat, high in fibre (Whole grains, legumes, vegetables and fruit) and ample fluids.
PROCESS OF CANCER DEVELOPMENT:
The fundamental abnormality resulting in the development of cancer is the continual unregulated proliferation of cancer cells. Rather than responding appropriately to the signals that control normal cell behavior, cancer cells grow and divide in an uncontrolled manner, invading normal tissues and organs and eventually spreading throughout the body. The generalized loss of growth control exhibited by cancer cells is the net result of accumulated abnormalities in multiple cell regulatory systems and is reflected in several aspects of cell behavior that distinguish cancer cells from their normal counterparts.
Cancer can result from abnormal proliferation of any of the different kinds of cells in the body, so there are more than a hundred distinct types of cancer, which can vary substantially in their behavior and response to treatment. The most important issue in cancer pathology is the distinction between benign and malignant tumors. A tumor is any abnormal proliferation of cells, which may be either benign or malignant. A benign tumor, such as a common skin wart, remains confined to its original location, neither invading surrounding normal tissue nor spreading to distant body sites. A malignant tumor, however, is capable of both invading surrounding normal tissue and spreading throughout the body via the circulatory or lymphatic systems (metastasis). Only malignant tumors are properly referred to as cancers, and it is their ability to invade and metastasize that makes cancer so dangerous. Whereas benign tumors can usually be removed surgically, the spread of malignant tumors to distant body sites frequently makes them resistant to such localized treatment.
Both benign and malignant tumors are classified according to the type of cell from which they arise. Most cancers fall into one of three main groups: carcinomas, sarcomas, and leukemias or lymphomas. Carcinomas, which include approximately 90% of human cancers, are malignancies of epithelial cells. Sarcomas, which are rare in humans, are solid tumors of connective tissues, such as muscle, bone, cartilage, and fibrous tissue. Leukemias and lymphomas, which account for approximately 8% of human malignancies, arise from the blood-forming cells and from cells of the immune system, respectively. Tumors are further classified according to tissue of origin (e.g., lung or breast carcinomas) and the type of cell involved. For example, fibrosarcomas arise from fibroblasts, and erythroid leukemias from precursors of erythrocytes (red blood cells). The four most common cancers, accounting for more than half of all cancer cases, are those of the breast, prostate, lung, and colon/rectum. Lung cancer, by far the most lethal, is responsible for nearly 30% of all cancer deaths.
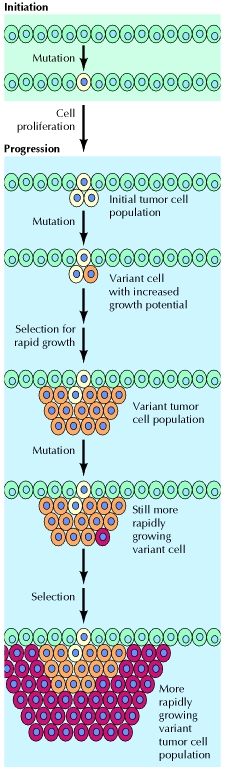
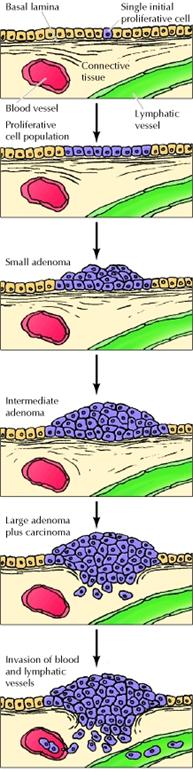
At the cellular level, the development of cancer is viewed as a multistep process involving mutation and selection for cells with progressively increasing capacity for proliferation, survival, invasion, and metastasis. The first step in the process, tumor initiation, is thought to be the result of a genetic alteration leading to abnormal proliferation of a single cell. Cell proliferation then leads to the outgrowth of a population of clonally derived tumor cells. Tumor progression continues as additional mutations occur within cells of the tumor population. Some of these mutations confer a selective advantage to the cell, such as more rapid growth, and the descendants of a cell bearing such a mutation will consequently become dominant within the tumor population. The process is called clonal selection, since a new clone of tumor cells has evolved on the basis of its increased growth rate or other properties (such as survival, invasion, or metastasis) that confer a selective advantage. Clonal selection continues throughout tumor development, so tumors continuously become more rapid-growing and increasingly malignant.
Studies of colon carcinomas have provided a clear example of tumor progression during the development of a common human malignancy. The earliest stage in tumor development is increased proliferation of colon epithelial cells. One of the cells within this proliferative cell population is then thought to give rise to a small benign neoplasm (an adenoma or polyp). Further rounds of clonal selection lead to the growth of adenomas of increasing size and proliferative potential. Malignant carcinomas then arise from the benign adenomas, indicated by invasion of the tumor cells through the basal lamina into underlying connective tissue. The cancer cells then continue to proliferate and spread through the connective tissues of the colon wall. Eventually the cancer cells penetrate the wall of the colon and invade other abdominal organs, such as the bladder or small intestine. In addition, the cancer cells invade blood and lymphatic vessels, allowing them to metastasize throughout the body.
Immunity and Tumor
The effector mechanisms of both cell-mediated immunity and humoral immunity have been shown to kill tumor cells in vitro. The challenge for tumor immunologists is to determine which of these mechanisms may contribute to protective immune responses against tumors and to enhance these effector mechanisms in ways that are tumor specific. In this section, we review the evidence for tumor killing by various immune effector mechanisms and discuss which are the most likely to be relevant to human tumors.
T-Lymphocytes:
The principal mechanism of tumor immunity is killing of tumor cells by CD8+ CTLs. The ability of CTLs to provide effective anti-tumor immunity in vivo is most clearly seen in animal experiments using carcinogen-induced and DNA virus-induced tumors. As discussed previously, CTLs may perform a surveillance function by recognizing and killing potentially malignant cells that express peptides derived from mutant cellular proteins or oncogenic viral proteins and presented in association with class I MHC molecules. The role of immune surveillance in preventing common, non-virally induced tumors has been questioned because such tumors do not arise more frequently in T cell-deficient animals or people. However, tumor-specific CTLs can be isolated from animals and humans with established tumors, such as melanomas. Furthermore, mononuclear cells derived from the inflammatory infiltrate in human solid tumors, called tumor-infiltrating lymphocytes (TILs), also include CTLs with the capacity to kill the tumor from which they were derived.
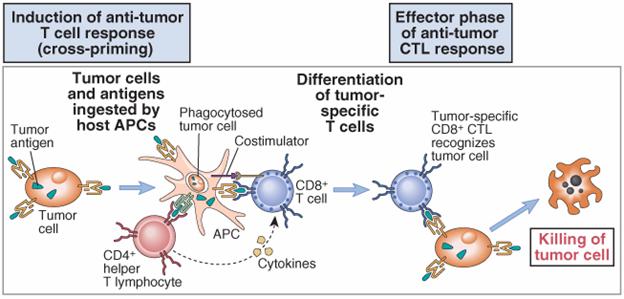
CD8+ T cell responses specific for tumor antigens may require cross-presentation of the tumor antigens by professional APCs, such as dendritic cells. Most tumor cells are not derived from APCs and therefore do not express the costimulators needed to initiate T cell responses or the class II MHC molecules needed to stimulate helper T cells that promote the differentiation of CD8+ T cells. A likely possibility is that tumor cells or their antigens are ingested by host APCs, particularly dendritic cells; the tumor antigens are then processed inside the APCs, and peptides derived from these antigens are displayed bound to class I MHC molecules for recognition by CD8+ T cells. The APCs express costimulators that may provide the signals needed for differentiation of CD8+ T cells into anti-tumor CTLs, and the APCs express class II MHC molecules that may present internalized tumor antigens and activate CD4+ helper T cells as well . Once effector CTLs are generated, they are able to recognize and kill the tumor cells without a requirement for costimulation. A practical application of the concept of cross-priming is to grow dendritic cells from a patient with cancer, incubate the APCs with the cells or antigens from that patient's tumor, and use these antigen-pulsed APCs as vaccines to stimulate anti-tumor T cell responses.
The importance of CD4+ helper T cells in tumor immunity is less clear. CD4+ cells may play a role in anti-tumor immune responses by providing cytokines for effective CTL development. In addition, helper T cells specific for tumor antigens may secrete cytokines, such as tumor necrosis factor (TNF) and interferon-γ (IFN-γ), that can increase tumor cell class I MHC expression and sensitivity to lysis by CTLs. IFN-γ may also activate macrophages to kill tumor cells. The importance of IFN-γ in tumor immunity is demonstrated by the finding of increased incidence of tumors in knockout mice lacking this cytokine.
Antibodies
Tumor-bearing hosts may produce antibodies against various tumor antigens. For example, patients with EBV-associated lymphomas have serum antibodies against EBV-encoded antigens expressed on the surface of the lymphoma cells. Antibodies may kill tumor cells by activating complement or by antibody-dependent cell-mediated cytotoxicity, in which Fc receptor-bearing macrophages or NK cells mediate the killing. However, the ability of antibodies to eliminate tumor cells has been demonstrated largely in vitro, and there is little evidence for effective humoral immunity against tumors.
NK cells
NK cells kill many types of tumor cells, especially cells that have reduced class I MHC expression and can escape killing by CTLs. In vitro, NK cells can kill virally infected cells and certain tumor cell lines, especially hematopoietic tumors. NK cells also respond to the absence of class I MHC molecules because the recognition of class I MHC molecules delivers inhibitory signals to NK cells. As we shall see later, some tumors lose expression of class I MHC molecules, perhaps as a result of selection against class I MHC-expressing cells by CTLs. This loss of class I MHC molecules makes the tumors particularly good targets for NK cells. In addition, NK cells can be targeted to IgG antibody-coated cells by Fc receptors (FcγRIII or CD16). The tumoricidal capacity of NK cells is increased by cytokines, including interferons and interleukins (IL-2 and IL-12), and the anti-tumor effects of these cytokines are partly attributable to stimulation of NK cell activity. IL-2-activated NK cells, called lymphokine-activated killer (LAK) cells, are derived by culturing peripheral blood cells or TILs from tumor patients with high doses of IL-2. The use of LAK cells in adoptive immunotherapy for tumors is discussed later.
The role of NK cells in tumor immunity in vivo is unclear. It has been suggested that T cell-deficient mice do not have a high incidence of spontaneous tumors because they have normal numbers of NK cells that serve an immune surveillance function. A few patients have been described with deficiencies of NK cells and an increased incidence of EBV-associated lymphomas.
Macrophages
The role of macrophages in
anti-tumor immunity is largely inferred from the demonstration that in vitro,
activated macrophages can kill many tumor cells more efficiently than they can
kill normal cells. How macrophages are activated by tumors is not known.
Possible mechanisms include direct recognition of some surface antigens of tumor
cells and activation of macrophages by IFN-γ produced by tumor-specific T cells.
Macrophages can kill tumor cells by several mechanisms, probably the same as the
mechanisms of macrophage killing of infectious organisms. These mechanisms
include the release of lysosomal enzymes, reactive oxygen intermediates, and
nitric oxide![]() .
Activated macrophages also produce the cytokine TNF, which was first
characterized, as its name implies, as an agent that can kill tumors mainly by
inducing thrombosis in tumor blood vessels.
.
Activated macrophages also produce the cytokine TNF, which was first
characterized, as its name implies, as an agent that can kill tumors mainly by
inducing thrombosis in tumor blood vessels.
Evasion of tumors from Immune system
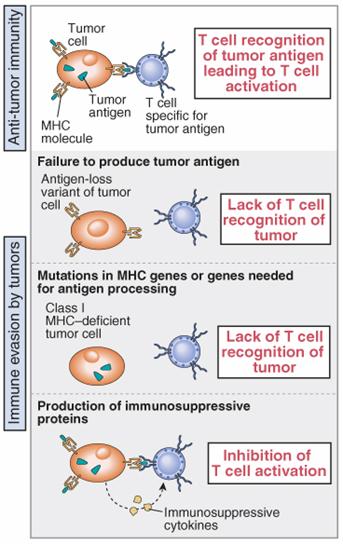
Many malignant tumors possess mechanisms that enable them to evade or resist host immune responses. A major focus of tumor immunology is to understand the ways in which tumor cells evade immune destruction, with the hope that interventions can be designed to increase the immunogenicity of tumors and the responses of the host. The process of evasion, often called tumor escape, may be a result of several mechanisms.
Immunotherapy
Immunotherapy for tumors is designed to augment active immune responses against these tumors or to administer tumor-specific immune effectors to patients. Immune responses may be actively enhanced by vaccination with tumor cells or antigens, administration of tumors modified to express high levels of costimulators or cytokines that stimulate T cell proliferation and differentiation, and systemic administration of cytokines. Approaches for passive immunotherapy include the administration of antitumor antibodies, antibodies conjugated with toxic drugs (immunotoxins), and tumor-reactive T cells and NK cells isolated from patients and expanded by culture with growth factors.
Dietary guidelines to lower cancer risk:
Dietary guidelines to lower cancer risk were developed by an expert panel on diet, nutrition and cancer convened by the National Academy of Sciences (NAS) in 1982. These guidelines are based on preliminary evidence.
Two types of studies are used to help identify cancer causing agents in foods: epidemiologic studies and laboratory tests of animals or humans. Epidemiologic cancer studies are comparisons between diet and death from cancer in certain populations. Laboratory studies are observations of animals or humans designed to test the effect of certain dietary factors.
Following the dietary guidelines to lower cancer risk does not guarantee that you will not get cancer. But the guidelines may help decrease your chances, particularly if you are at increased risk because of genetic or other reasons. These guidelines also may lessen the risk of other chronic diseases. These cancer recommendations generally conform to the USDA and U.S. Health and Human Services Dietary Guidelines for Americans. The dietary guidelines to lower cancer risk are:
These guidelines are not intended to overly restrict or eliminate any foods from your diet. They are meant to encourage you to consume a wide variety of foods. Choosing certain types of foods more often than others also is encouraged, as suggested below.
Guideline #1
Reduce intake of dietary fat -- saturated and unsaturated -- from the current average of approximately 34 percent to a level of 30 percent of total calories.
Fat in the diet. Laboratory and epidemiologic data suggests that too much fat in the diet leads to an increased risk of a variety of cancers. The risk of these cancers appears to be higher when fat as a total percentage of calorie intake is increased. Americans consume an average of 34 percent of their calories as fat. The Food and Nutrition Board of the National Academy of Sciences recommends that people eat no more than 30 percent of their daily calories as fat.
Tips to reduce fat in your diet. Suggestions to cut down on the fat in your diet include:
Guideline #2
Increase consumption of fruits, vegetables and whole grain cereals.
Specific nutrients and food constituents of fruits, vegetables and whole grain cereals may be anticancer substances when eaten at levels found in a varied diet. Some food constituents and specific nutrients believed to protect against cancer are dietary fiber, phytochemicals, and vitamins A, C and E.
Dietary fiber. Dietary fiber is the material from plant cells that the body cannot digest completely. Dietary fiber is found in vegetables, legumes, fruit and whole-grain cereals, nuts and seeds. A diet high in fiber and low in fat may reduce the risk of colon and rectal cancers. Fiber provides bulk in the diet, and it helps move food through the intestines and out of the body at regular intervals. It is unclear whether it is total fiber intake or the components of dietary fiber that is beneficial in reducing cancer risks.
Phytochemicals. Evidence suggests that certain compounds in plant food sources, particularly fruits and vegetables, can be cancer fighting. These compounds are called phytochemicals, which is actually a general name for several cancer-fighting substances, such as flavonoids, allylic sulfides and sulforaphanes. Scientists believe that the compounds stop cancer cells from initiating or developing into tumors. While there is no direct proof these compounds can prevent cancer, eating a diet with plenty of fruits and vegetables has proven to be a healthy food choice. Obtaining anticancer substances from food rather than supplements may be the best plan of action to prevent disease.
Beta carotene and Vitamin A. Our bodies convert beta carotene to vitamin A. Epidemiological studies suggest that eating foods that contain beta carotene decreases the risk of a variety of cancers. Supplements are not found to provide the same benefit, and excess vitamin A can be toxic. Get vitamin A naturally by eating vitamin A or beta carotene-rich fruits and vegetables: dark green and yellow vegetables such as carrots, winter squash, broccoli and spinach. Deep yellow-orange fruits, such as apricots, peaches and cantaloupe, also are good sources.
Vitamin C. Vitamin C-rich foods may have cancer-inhibiting benefits, particularly for cancers of the stomach and esophagus. Nitrites and nitrates occur naturally in foods. They are commonly added to foods as preservatives and to processed meats for color. These substances can combine with amino acids from proteins to form nitrosamines. Nitrosamines are known to cause cancer. This reaction definitely occurs in the test tube. Whether it occurs in the human digestive tract is not yet clear. Research has demonstrated that vitamin C (ascorbic acid) can inhibit the reaction of nitrites with amines or amides. It competes with the amine for the nitrite, which inhibits carcinogenic compound formation.
Vitamin E. Vitamin E has been shown to protect against cancer in some experimental animal studies. The mechanism is similar to vitamin C's: it competes for available nitrite, which blocks the formation of nitrosamines. However, epidemiologic data are conflicting (Nurses’Health Study). It is better to get Vitamin E from food sources.
Cruciferous vegetables. Cruciferous vegetables are from the cabbage family and include bok-choy, broccoli, brussels sprouts, cabbage, cauliflower, collards, kale, kohlrabi, mustard greens and turnips. These vegetables also may be important in reducing the risk of cancer, particularly cancer of the gastrointestinal and respiratory tracts. Scientists believe the anticancer compound in these foods is called sulforaphane. It is unclear what component of these vegetables is responsible for reducing the risk of cancer, but studies have demonstrated their protective effect.
Tips to increase consumption. It is important to eat a variety of vitamin- and mineral-rich foods, especially fruits, vegetables and whole-grain breads and cereals, rather than relying on supplements. There may be undiscovered cancer-protecting components or nutrients that occur naturally in foods. Also, eating a variety of foods will provide adequate vitamins and minerals to maintain health. To increase dietary fiber, vitamins and selenium, select fiber-rich, whole-grain breads, cereals and pastas; all varieties of fruits and vegetables; legumes; and oat-based products. An added benefit of eating more carbohydrate foods is that fat intake generally is decreased.
Guideline #3
Consume salt-cured, smoked and charcoal broiled foods only in moderation.
Carcinogens are present in certain foods. Evidence suggests that eating salt-cured, smoked, pickled and charcoal-broiled foods increases cancer risk. In parts of the world where food is often prepared using these methods, stomach and esophageal cancer cases are higher. In the United States, stomach cancer is declining and esophageal cancer is rare.
Nitrates, often used in the curing process, cause cancer in laboratory animals and are suspected of causing cancer in people. In the process of smoking foods, the foods absorb large amounts of tar that arise from incomplete combustion of wood or charcoal fire. These tars have been found to contain numerous carcinogens. Today, "liquid smoke," which may be less hazardous, often is used.
When meats are charcoal- or gas-broiled, a substance (benzopyrene) is formed when fat from the meat drips onto the hot coals. The rising smoke then carries this carcinogenic substance back up and deposits it onto the meat. However, little evidence suggests that Americans are at risk from excessive consumption of charcoal-broiled food. Much research still is needed to determine the links between charcoal-broiled foods and cancer. In the meantime, it makes sense to consume these foods in moderation.
High-temperature frying or broiling, for example frying bacon, may convert some of the meat proteins into products that damage the genetic material of the body's cells.
Tips to eat moderate amounts. To reduce cancer risk and still enjoy a cookout, cover the grill with foil and punch holes between the grids to let the fat drip out. The foil protects food from smoke and fire. Cook meat until done but don't char it. If food does get charred, remove the charred portions before eating it. Discourage flareups by either dampening coals that become too hot with a squirt of water, or move food to another section of the grill. Also, reduce cooking time on the grill by partially precooking foods in a microwave and then grilling briefly to give it that grilled flavor. It's probably a good idea to eat salt-cured and smoked foods only once in awhile.
Guideline #4
Drink alcoholic beverages only in moderation.
Alcohol abuse, diet and cancer. Heavy drinking of alcoholic beverages, more than two drinks per day, increases the risk of mouth, pharynx, larynx, esophagus, liver, pancreas and bladder cancers. It is unclear whether it is alcohol or other ingredients in these beverages that are responsible for the association with cancer in people. The carcinogenic effect may be the direct contact of alcohol on the mouth, pharynx and esophagus.
Heavy drinking can result in liver cirrhosis, which may lead to liver cancer. Heavy drinkers and alcoholics commonly have nutritional deficiencies because alcohol contains only empty calories, and food intake often is compromised. When little food is eaten, low nutrient intake often results. If heavy drinkers smoke, as is commonly the case, cancer risk escalates.
The link between cancer and alcohol is complex because frequent alcohol consumption may result in many health problems. The nutritional cancer risk factors are compounded for alcohol abusers. Alcohol is high in calories and low in nutrients. It is difficult for an alcohol abuser to obtain protective benefits from foods when so little nutrient-dense food is eaten.
Tips for moderate alcohol consumption. Instead of alcohol, try non-alcoholic wine, beer, mineral or tonic water, cider, grape juice, or fruit juice. Always provide nonalcoholic beverages and nutrient-dense foods at social gatherings. If you do drink, do so in moderation -- less than two drinks per day -- and don't drive.
Cancer Diet
There is a lot you can do to give yourself the best chance to win the fight against cancer. Staying nutritionally fortified is one positive way to take control of your life and your well- being. Optimal nutrition allows your body to function at its best. Maintaining optimal nutrition can provide several benefits for people living with cancer, including:
Good nutrition is essential to keep you strong—to increase the chance that your cancer treatment goes uninterrupted. Your body needs more "fuel" than normal during this time, because it needs to repair from the effects of cancer treatments, such as surgery, radiation therapy and/or chemotherapy. If you are unable to consume the fuel you need, your body will soon draw upon what it has stored—fat and protein. When your body uses stored protein, malnutrition and impaired functioning of your immune system may result.
According to the National Cancer Institute, about one-third of all cancer deaths are related to malnutrition. Therefore, it is important to give your body a constant supply of nutrients to use as fuel during the healing process. This supply of nutrients includes calories from all macronutrients, including carbohydrates, protein and fat.