IMMUNITY
Immunity refers to the ability of immune system to defend against diseases caused by microbes or foreign substances. Immunity depends upon various factors like host resistance, dosage of organism injected and virulence of the organisms. The power of resistance to infection may be natural i.e., it may be inherited or innate or it may be acquired as a result of previous infection or by inoculation. Immunity is the state of protection against disease, usually infectious disease, mediated by a collection of molecules, cells and tissues collectively called the immune system.
HISTORY OF IMMUNITY:
The first recorded crude attempts to deliberately induce immunity were performed by the Chinese and Turks in the fifteenth century. Various reports suggest that the dried crusts derived from small pox pustles were either inhaled into the nostrils or inserted into small cuts in the skin. It induces immunity against smallpox. The process is known as variolation.
Lady Mary Wortley Montagu, the wife of the British ambassador to Constantinople, observed the positive effects of variolation on the native population and had the technique applied to her own children. This method significantly improved by Edward Jenner. Milkmaids who contracted cowpox were subsequently immune to smallpox; Jenner reasoned that introducing fluid from a cowpox pustule into people might protect them from smallpox.
To test this idea, he inoculated an 8 year old boy with fluid from a cowpox pustule and later intentionally infected the child with smallpox. The next major advance in immunology was the induction of immunity to cholera by Louis Pasteur.
THEORIES OF IMMUNITY:
There are three theories considered as main for immunity. They are selective theory, Instructional theory and clonal selection theory.
CLASSIFICATION:
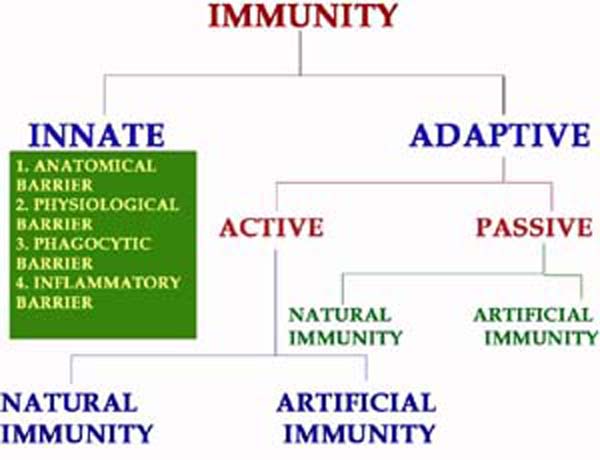
Immunity mainly classified into innate and acquired immunity. Innate or nonspecific immunity refers to the basic resistance to disease that an individual is born with. Acquired or specific or adaptive immunity requires the activity of a functional immune system, involving cells called lymphocytes and their products. Innate defense mechanisms provide the first line of defense against invading pathogens until an acquired immune response develops. In general, most of the microorganisms encountered by healthy individual are readily cleared within a few days by non-specific defense mechanisms without enlisting a specific immune response. When an invading microorganism eludes the nonspecific host defense mechanism, a specific immune response then in enlisted. Innate immunity provided by anatomic, physiologic, phagocytic and inflammatory barriers.

|
S.No |
Characteristics & Components |
Innate Immunity |
Adaptive Immunity |
| 1. | Specificity | For structures shared by groups of related microbes | For antigens of microbes and for non-microbial antigens |
| 2. | Diversity | Limited; Germline encoded | Very large; receptors are produced by somatic recombination of gene segments |
| 3. | Memory | None | Yes |
| 4. | Non reactivity to self | Yes | No |
| 5. | Physical and Chemical Barriers | Skin, mucosal epithelia; antimicrobial chemicals | Lymphocytes in epithelia; antibodies secreted at epithelial surfaces |
| 6. | Blood Proteins | Complement | Antibodies |
| 7. | Cells | Phagocytes, Natuarl Killer cells | Lymphocytes |
Acquired immunity further classified into Passive and Active immunity. Passive immunity is the form of immunity to an antigen that is established in one individual by transfer of antibodies or lymphocytes from another individual who is immune to that antigen. The recipient of such a transfer can become immune to the antigen without even having been exposed to or having responded to the antigen. In this case, the recipient's immune system plays no active role. There is no antigenic stimulus; instead, preformed antibodies are administered. There is no latent period in passive immunity, protection being effective immediately following passive immunization. There is no negative phase. The immunity is transient, lasting usually for days or weeks only till the passively transmitted antibodies are metabolized and eliminated. No secondary type response occurs in passive immunity. Passive immunity intern are of two types namely Natural Passive immunity and Artificial Passive immunity. Natural Passive immunity is provided to the fetus through antibodies transferred from mother to fetus through placenta. The immune component transferred in this process is known as “Transfer factor”.Artificial Passive immunity can be induced in individual against snake venom by the transfer of human sera containing antibodies specific for snake venom to a previously unimmunized individual. In fact, passive immunity diminishes in effect with repetition. When a foreign antibody is administered a second time, it is eliminated more rapidly than initially. Following the first injection of an antibody, e.g., immune horse serum, the elimination is only by metabolic breakdown but during subsequent injections of horse serum, its elimination is much quicker as it combines with antibodies to horse serum that would have been produced following its initial injection. This is referred as immune elimination. This factor of immune elimination limits the usefulness of repeated passive immunization.
Active immunity is the form of adaptive immunity that is induced by exposure to a foreign antigen and activation of lymphocytes and in which the immunized individual plays an active role in responding to the antigen. It is also further divided into Natural active immunity and artificial active immunity. Natural active immunity provided when an individual exposed to antigen naturally. A person who has recovered from an attack of measles develops natural active immunity. Artificial active immunity provided by vaccines. Vaccines are preparations of live attenuated or killed microorganisms or their products used for immunization.
|
S.No |
Active Immunity |
Passive Immunity |
|
1. |
Produced actively by the host's immune system. |
Received passively by the host. No participation by the host's immune system. |
|
2. |
Induced by infection or by contact with immunogens. |
Conferred by introduction of readymade antibodies. |
|
3. |
Affords durable and effective protection. |
Protection transients and less effective. |
|
4. |
Immunity effective only after a lag period i.e., time required for generation of antibodies. |
Immunity effective immediately. |
|
5. |
Immunological memory present. Subsequent challenge more effective. This is known as booster effect. |
No immunologic memory; subsequent administration of antibody less effective due to immune elimination. |
|
6. |
Negative phase may occur. |
No negative phase. |
|
7. |
Not applicable in immunodeficient hosts. |
Applicable in immunodeficient hosts. |
In some cases, immunity present only when original infection remains active. Such immunity is known as premunition. In syphilis, the immunity to reinfection lasts only as long as the original infection remains active. Once disease is cured, the patient becomes susceptible to the spirochete again.
Depending upon the component involved in immunity, specific immunity further divided into two types namely Humoral immunity and cell mediated immunity. Cell mediated immunity is the form of adaptive immunity that is mediated by T lymphocytes and serves as the defense mechanism against microbes that survive within phagocytes or infect non-phagocytic cells. Cell mediated Immunity (CMI) responses include TH cell mediated activation of macrophages that have phagocytosed microbes and TC cells killing of infected cells through cytotoxicity reactions. Humoral Immunity is mediated by antibodies produced by B lymphocytes. Humoral immunity is the principal defense mechanism against extracellular microbes and their toxins.
If Immunity is achieved by transferring immunologically competent lymphocytes then such immunity is known as adoptive immunity. The immunologically competent lymphocytes are known as transfer factor. This type of immunity has been attempted in the therapy of certain types of diseases. One of the examples is lepromatous leprosy.
The concept of local immunity, proposed by Besredka, has gained importance in the treatment of infections which are either localized or where it is operative in combating infection at the site of primary entry of the pathogen. In poliomyelitis, for instance, systemic immunity provided by active immunization with the killed vaccine neutralizes the virus when it enters the bloodstream. But it does not prevent multiplication of the virus at the site of entry and its fecal shedding. This is achieved by local intestinal immunity acquired as a result of either natural infection or immunization with the live oral vaccine. In influenza, immunization with the killed vaccine elicits a humoral antibody response. But the antibody titer in respiratory secretions is often not high enough to prevent infection. Natural infection or the live virus vaccine administered intranasally provides local immunity. A special class of immunoglobulins, Ig A forms major component of local immunity. Because of this mucosal immune system is otherwise known as secretory immune system.
Immunity present in a community or herd is known as herd immunity. It is relevant in the control of epidemic diseases. When a large proportion of individuals in a community are immune to a pathogen, the herd immunity to the pathogens is satisfactory. When herd immunity is low, epidemics are likely to occur on the introduction of a suitable pathogen, due to the presence of large numbers of susceptible individuals in the community. Eradication of communicable diseases depends on the development of a high level of immunity in individuals.
INNATE IMMUNITY:
Innate immunity may be considered at the level of the species, race or individual. Species immunity refers to the total or relative refractoriness to a pathogen, shown by all members of a species. For instance, all human beings are totally insusceptible to plant pathogens and to many pathogens of animals such as rinderpest or distemper. This immunity is something a person obtains as a birthright, for the reason that he belongs to the human species. The mechanisms of species immunity are not clearly understood but may be due to physiological and biochemical differences between the tissues of the different host species, which determine whether or not a pathogen can multiply in them. An early insight into the basis of species immunity was gained by Pasteur's experiments on anthrax in frogs, which are naturally resistant to the disease but become susceptible when their body temperature is raised from 25* C to 35* C.
Within a species, different races may show differences in susceptibility to infections. This is known as racial immunity, the classic example of which is the high resistance of Algerian sheep to anthrax. Such racial differences are known to be genetic in origin, and by selection and inbreeding, it is possible to develop, at will, races that possess high degrees of resistance or susceptibility to various pathogens. It has been reported that the Negroes in the USA are more susceptible than the whites to tuberculosis. The differences in innate immunity exhibited by different individuals in a race are known as individual immunity. It is well documented that homozygous twins exhibit similar degrees of resistance or susceptibility to lepromatous leprosy and tuberculosis.
Innate immunity provided by four types of defensive barriers namely anatomic, physiologic, endocytic and inflammatory barriers.
ANATOMIC BARRIERS:
Physical and anatomic barriers that tend to prevent the entry of pathogens are an organism’s first line of defense against infection. The skin and the surface of mucous membranes are included in this category because they are effective barriers to the entry of most microorganisms. The skin consists of two distinct layers: a thinner outer layer—the epidermis—and a thicker layer—the dermis. The epidermis contains several layers of tightly packed epithelial cells. The outer epidermal layer consists of dead cells and is filled with a waterproofing protein called keratin. The dermis, which is composed of connective tissue, contains blood vessels, hair follicles, sebaceous glands, and sweat glands. The sebaceous glands are associated with the hair follicles and produce an oily secretion called sebum. Sebum consists of lactic acid and fatty acids, which maintain the pH of the skin between 3 and 5; this pH inhibits the growth of most microorganisms. A few bacteria that metabolize sebum live as commensals on the skin and sometimes cause a severe form of acne. One acne drug, isotretinoin (Accutane), is a vitamin A derivative that prevents the formation of sebum. Breaks in the skin resulting from scratches, wounds, or abrasion are obvious routes of infection. The skin may also be penetrated by biting insects (e.g., mosquitoes, mites, ticks, fleas, and sandflies); if these harbor pathogenic organisms, they can introduce the pathogen into the body as they feed. The protozoan that causes malaria, for example, is deposited in humans by mosquitoes when they take a blood meal. Similarly, bubonic plague is spread by the bite of fleas, and Lyme disease is spread by the bite of ticks. The conjunctivae and the alimentary, respiratory, and urogenital tracts are lined by mucous membranes, not by the dry, protective skin that covers the exterior of the body. These membranes consist of an outer epithelial layer and an underlying layer of connective tissue. Although many pathogens enter the body by binding to and penetrating mucous membranes, a number of nonspecific defense mechanisms tend to prevent this entry. For example, saliva, tears, and mucous secretions act to wash away potential invaders and also contain antibacterial or antiviral substances. The viscous fluid called mucus, which is secreted by epithelial cells of mucous membranes, entraps foreign microorganisms. In the lower respiratory tract, the mucous membrane is covered by cilia, hair like protrusions of the epithelial-cell membranes. The synchronous movement of cilia propels mucus-entrapped microorganisms from these tracts. In addition, nonpathogenic organisms tend to colonize the epithelial cells of mucosal surfaces. These normal flora generally out compete pathogens for attachment sites on the epithelial cell surface and for necessary nutrients.
PHYSIOLOGIC BARRIERS:
The physiologic barriers that contribute to innate immunity include temperature, pH, and various soluble and cell associated molecules. Many species are not susceptible to certain diseases simply because their normal body temperature inhibits growth of the pathogens. Chickens, for example, have innate immunity to anthrax because their high body temperature inhibits the growth of the bacteria. Gastric acidity is an innate physiologic barrier to infection because very few ingested microorganisms can survive the low pH of the stomach contents. One reason newborns are susceptible to some diseases that do not afflict adults is that their stomach contents are less acid than those of adults. A variety of soluble factors contribute to innate immunity, among them the soluble proteins lysozyme, interferon, and complement. Lysozyme, a hydrolytic enzyme found in mucous secretions and in tears, is able to cleave the peptidoglycan layer of the bacterial cell wall. Interferon comprises a group of proteins produced by virus-infected cells. Complement lysed bacteria by forming membrane attack complex.
PHAGOCYTIC BARRIERS:
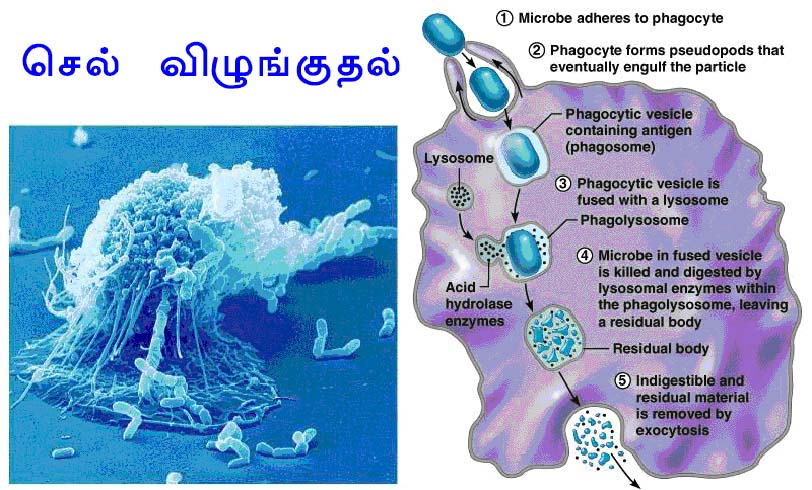
Another important innate defense mechanism is the ingestion of extracellular particulate material by phagocytosis. Phagocytosis is one type of endocytosis, the general term for the uptake by a cell of material from its environment. In phagocytosis, a cell’s plasma membrane expands around the particulate material, which may include whole pathogenic microorganisms, to form large vesicles called phagosomes. Most phagocytosis is conducted by specialized cells, such as blood monocytes, neutrophils, and tissue macrophages. Most cell types are capable of other forms of endocytosis, such as receptor-mediated endocytosis, in which extracellular molecules are internalized after binding by specific cellular receptors, and pinocytosis, the process by which cells take up fluid from the surrounding medium along with any molecules contained in it.
The process of phagocytosis was discovered by Metchnikoff. The term phagocytic denotes the engulfment and digestion of whole cells. The two major cell types in the body which are associated with the engulfment and digestion of microorganism are the polymorphonuclear leucocytes and the macrophages. Minor cell types are eosinophils. The process of phagocytosis involves the following steps:
1. Attachment:
Attachment is the adherence of bacterium to the cell membrane of the phagocytic cell. Some bacteria are easily attached to the phagocytic cell example mycobacterium tubercle. Some other requires that they be coated with antibody before attachment. This is called opsonization. Thus, antibody functions as an opsonin, a molecule that binds to both antigen and macrophage and enhances phagocytosis.
2. Phagosome formation:
After attachment the phagocyte extent small pseudopodia around the infecting bacterium, the pseudopodia fuse to form a pouch which contain a bacterium surrounded by the cell membrane. This structure is called as the phagosome.
3. Phagolysosome formation:
After engulfment in the form of phagosome, the lysosome containing the hydrolytic enzymes fuses with phagosome to form phagolysosome. In this step, lysosomal enzymes discharged to phagosome which is vital for the lysis of bacteria.
4. Lysis:
A number of antimicrobial and cytotoxic substances produced by activated macrophages can destroy phagocytosed microorganisms.
OXYGEN-DEPENDENT KILLING MECHANISMS:
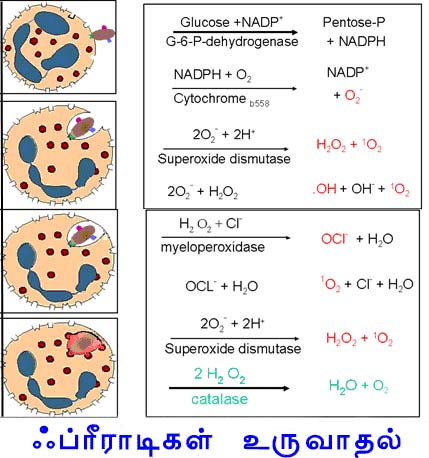
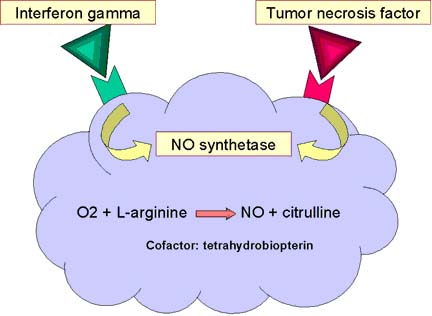
Activated phagocytes produce a number of reactive oxygen intermediates (ROIs) and reactive nitrogen intermediates that have potent antimicrobial activity. During phagocytosis, a metabolic process known as the respiratory burst occurs in activated macrophages. This process results in the activation of a membrane-bound oxidase that catalyzes the reduction of oxygen to superoxide anion, reactive oxygen intermediate that is extremely toxic to ingested microorganisms. The superoxide anion also generates other powerful oxidizing agents, including hydroxyl radicals and hydrogen peroxide. As the lysosome fuses with the phagosome, the activity of myeloperoxidase produces hypochlorite from hydrogen peroxide and chloride ions. Hypochlorite, the active agent of household bleach, is toxic to ingested microbes. When macrophages are activated with bacterial cell-wall components such as lipopolysaccharide (LPS) or, in the case of mycobacteria, muramyl dipeptide (MDP), together with a T-cell–derived cytokine (IFN-g), they begin to express high levels of nitric oxide synthetase (NOS), an enzyme that oxidizes L-arginine to yield L-citrulline and nitric oxide (NO), a gas:
L-arginine + O2 +NADPH/H+ → NO + L-citrulline + NADP+
Nitric oxide has potent antimicrobial activity; it also can combine with the superoxide anion to yield even more potent antimicrobial substances. Recent evidence suggests that much of the antimicrobial activity of macrophages against bacteria, fungi, parasitic worms, and protozoa is due to nitric oxide and substances derived from it.
OXYGEN-INDEPENDENT KILLING MECHANISMS:
Activated macrophages also synthesize lysozyme and various hydrolytic enzymes whose degradative activities do not require oxygen. In addition, activated macrophages produce a group of antimicrobial and cytotoxic peptides, commonly known as defensins. Cathepsin G is an example for defensins. These molecules are cysteine-rich cationic peptides containing 29–35 amino-acid residues. Each peptide, which contains six invariant cysteines, forms a circular molecule that is stabilized by intramolecular disulfide bonds. These circularized defensin peptides have been shown to form ion-permeable channels in bacterial cell membranes. Defensins can kill a variety of bacteria, including Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, Pseudomonas aeruginosa, and Haemophilus influenzae. Activated macrophages also secrete tumor necrosis factor (TNF), a cytokine that has a variety of effects and is cytotoxic for some tumor cells. Lactoferin chelates iron from the medium and prevent the growth and proliferation of iron dependent bacteria. Lysozyme splits mucopeptide in bacterial cell wall and lysed bacteria.
5. Exocytosis:
Finally the killed organisms are digested by hydrolytic enzymes and the degraded products are released to the exterior by the process of exocytosis.
Toll like Receptors (TLRs) are homologous to drosophila protein called Toll and function to activate phagocytes in response to different types and components of microbes. To date, 10 mammalian TLRs have been identified and each appears to be required for responses to a different class of infectious pathogen. Different TLRs play essential roles in cellular responses to lipopolysaccharides, other bacterial proteoglycans and unmethylated CpG nucleotides, all of which are found only in bacteria. These receptors function by receptor associated kinases to stimulate the production of microbial substances and cytokines in the phagocytes. TLRs may associate with other molecules that participate in the direct recognition of microbial products. This property is known as pattern recognition i.e., the ability to recognize a given class of molecules.
Reticulo Endothelial System (RES):
If an animal is intravenously injected with a suspension of carbon particles such as those found in India ink, the particles are rapidly removed from the blood by phagocytic cells distributed throughout the body. Ludwig Aschoff, the German scientist who discovered this phenomenon, believed that these cells collectively formed a body system, which he called the reticulo endothelial system. Subsequent investigations showed, however, that this was not the case. The so-called RES was a mixture of cells and system. Some of the cells that take up carbon particles do so inadvertently and not as their prime function; these include epidermal cells, intestinal epithelial cells and vascular endothelial cells.
Some are Neutrophils of the myeloid system. Nevertheless, among the cells that take up particulate matter these are professional phagocytes whose major function is the removal of foreign material and cell debris from tissues and blood. These professional phagocytes form a well defined body system called the mononuclear phagocytic system.
The mononuclear phagocytic system consists of a single population of cells called macrophages with similar morphology, common function and common origin. Macrophages usually have a single, rounded nucleus and can therefore be readily distinguished from neutrophils. They are avidly phagocytic hence they are mononuclear phagocytes. In contrast to neutrophils, macrophages are capable of sustained, repeated phagocytic activity. This is however, just one of their functions. They also process antigen so that it can stimulate immune response; they control inflammation and healing; and they remove dead, dying and damaged tissues. In different tissues macrophages called in different names they are as follows:
|
S.No |
Tissue location |
Name |
|
1. |
Brain |
Microglial cells |
|
2. |
Liver |
Kupffer cells |
|
3. |
Connective tissue |
Histiocytes |
|
4. |
Lung |
Alveolar macrophage |
|
5. |
Bone marrow |
Promonocytes |
|
6. |
Blood |
Monocytes |
|
7. |
Kidney |
Mesengial cells |
INFLAMMATORY BARRIERS:
This barrier created by the inflammatory response. Tissue damage caused by a wound or by an invading pathogenic microorganism induces a complex sequence of events collectively known as the inflammatory response. Inflammatory response described by the "four cardinal signs of inflammation" as rubor (redness), tumor (swelling), calor (heat) and dolor (pain). Presently fifth sign functiolaesa (loss of function) included.
The cardinal signs of inflammation reflect the three major events of an inflammatory response:
1. Vasodilation nearby capillaries occurs as the vessels that carry blood away from the affected area constrict, resulting in engorgement of the capillary network. The engorged capillaries are responsible for tissue redness (erythema) and an increase in tissue temperature.
2. An increase in capillary permeability facilitates an influx of fluid and cells from the engorged capillaries into the tissue. The fluid that accumulates (exudate) has much higher protein content than fluid normally released from the vasculature. Accumulation of exudate contributes to tissue swelling (edema).
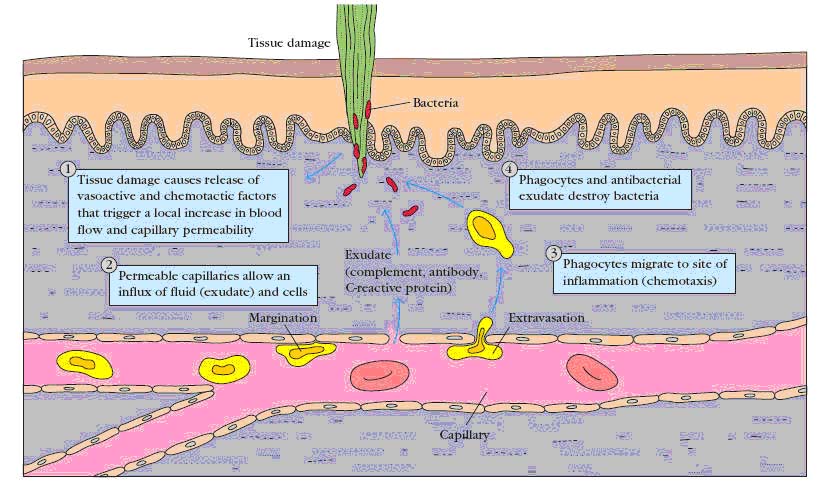
3. Influx of phagocytes from the capillaries into the tissues is facilitated by the increased permeability of the capillaries. The emigration of phagocytes is a multistep process that includes adherence of the cells to the endothelial wall of the blood vessels (margination), followed by their emigration between the capillary endothelial cells into the tissue (diapedesis or extravasation), and, finally, their migration through the tissue to the site of the invasion (chemotaxis). As phagocytic cells accumulate at the site and begin to phagocytose bacteria, they release lyric enzymes, which can damage nearby healthy cells. The accumulation of dead cells, digested material, and fluid forms a substance called pus.
Cell adhesion molecules (CAMs):
Endothelial cells express leukocyte-specific cell adhesion molecules (CAMs). Some of these membrane proteins are expressed constitutively; others are expressed only in response to local concentrations of cytokines produced during an inflammatory response. Most of these CAMs belong to four families of proteins: the selectin family, the mucin-like family, the integrin family, and the immunoglobulin (Ig) super family.
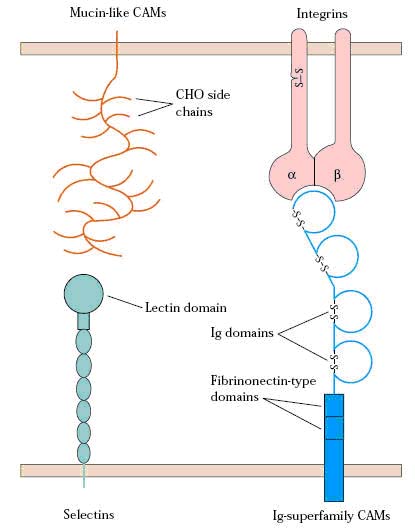
SELECTINS:
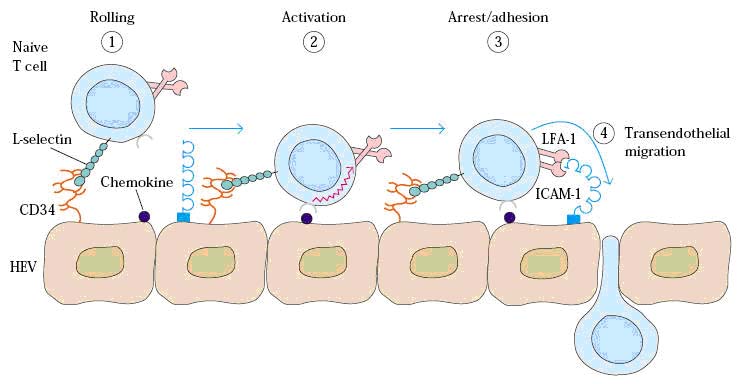
The selectin family of membrane glycoproteins has a distal lectin-like domain that enables these molecules to bind to specific carbohydrate groups. Selectins interact primarily with sialylated carbohydrate moieties, which are often linked to mucin-like molecules. The selectin family includes three molecules, designated L, E, and P. Most circulating leukocytes express L-selectin, whereas E-selectin and P-selectin are expressed on vascular endothelial cells. Selectin molecules are responsible for the initial stickiness of leukocytes to vascular endothelium.
MUCINS:
The mucins are a group of serine- and threonine rich proteins that are heavily glycosylated. Their extended structure allows them to present sialylated carbohydrate ligands to selectins. For example, L-selectin on leukocytes recognizes sialylated carbohydrates on two mucin-like molecules (CD34 and GlyCAM-1) expressed on certain endothelial cells of lymph nodes. Another mucin-like molecule (PSGL-1) found on neutrophils interacts with E- and P-selectin expressed on inflamed endothelium. Ex: GlyCAM-1, CD34, PSGL-1 and MAdCAM-1
INTEGRINS:
The integrins are heterodimeric proteins (consisting of an a and a b chain) that are expressed by leukocytes and facilitate both adherence to the vascular endothelium and other cell-to-cell interactions. The integrins are grouped into categories according to which b subunit they contain. Different integrins are expressed by different populations of leukocytes, allowing these cells to bind to different CAMs that belong to the immunoglobulin super family expressed along the vascular endothelium. Ex: VLA4; LFA1;
ICAMS or Ig Super family:
Several adhesion molecules contain a variable number of immunoglobulin-like domains and thus are classified in the immunoglobulin super family. Included in this group are ICAM-1, ICAM-2, ICAM-3, and VCAM, which are expressed on vascular endothelial cells and bind to various integrin molecules. An important cell-adhesion molecule called MAdCAM-1 has both Ig-like domains and mucin-like domains. This molecule is expressed on mucosal endothelium and directs lymphocyte entry into mucosa. It binds to integrins by its immunoglobulin-like domain and to selectins by its mucin-like domain.
Cell adhesion molecules play important role in extravasation. Some of these adhesion molecules are distributed in a tissue specific manner. These tissue specific adhesion molecules have been called vascular addressins because they direct different lymphocytes to different lymphoid organs.
High Endothelial Venules (HEV):
Some regions of vascular endothelium in postcapillary venules of various lymphoid organs are composed of specialized cells with a plump, cuboidal (“high”) shape; such regions are called high-endothelial venules, or HEVs. Their cells contrast sharply in appearance with the flattened endothelial cells that line the rest of the capillary. Each of the secondary lymphoid organs, with the exception of the spleen, contains HEVs. The process of migration of different subsets of lymphocytes differently into different tissues is known as Homing or trafficking. The different trafficking achieved due to the presence of different vascular addressins on HEVs, they are referred as Homing receptors.
Note: Shutdown phase: After an antigen encountered, Naive lymphocytes trapped in secondary lymphoid organ for activation for about 48 hours. At this time, antigen specific lymphocytes can not be detected in the circulation like lymph. This is known as shut down phase.
Steps in Extravasation of T-cells:
MEDIATORS OF INFLAMMATION:
Inflammatory mediators are released by tissue mast cells, blood platelets and a variety of leucocytes including neutrophils, monocytes / macrophages, eosinophils, basophils and lymphocytes.
1. Chemokines:
Chemokines like Macrophage inflammatory protein 1a, Macrophage inflammatory protein 1b, RANTES, IL- 8, Neutrophil activating protein -2, and platelet factor -4, C-C sub group chemokines and C – X – C subgroup chemokines are released during inflammation.
2. Plasma enzyme mediators:
They include kinin system, the clotting system, fibrinolytic system and the complement system.
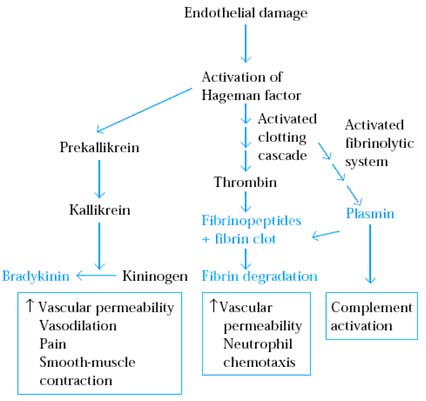
a. Kinin System:
Activation of Hagman factor is the first step of kinin system. The activated Hagman factor activates prekallikrein to kallikrein which intern forms bradykinin from kininogen. Bradykinin increases vascular permeability, causes vasodilation, induces pain and induces smooth muscle contraction. Kallikrein also acts directly on the complement system by cleaving C5 into C5a and C5b. The C5a, an anaphylatoxins induces mast cell degradulation which results in the release of number of inflammatory mediators.
b. Clotting System:
The clotting system is triggered very rapidly following tissue injury to prevent bleeding and limit spread of invading pathogens into the blood stream. Through clotting system, thrombin formed from prothrombin. Thrombin acts on soluble fibrinogen in tissue fluid plasma to produce insoluble strands of fibrin and fibrnopeptides. The insoluble fibrin strands crisscross one another forming a clot which serves as a barrier to the spread of infection. This mechanism is referred as “walling off” reaction. The fibrnopeptides act inflammatory mediators inducing increased vascular permeability and neutrophil chemotaxis.
c. Fibrinolytic System:
Fibrinolytic system removes clot. The end product of this pathway is the enzyme plasmin, which is formed from plasminogen. Plasmin breaks down fibrin clots into degradation products that are chemotactic for neutrophils. Plasmin also contributes to the inflammatory response activating the classical complement pathway.
d. Complement System:
Complement pathways release activated complement intermediates. Of these C3a, C4a and C5a are known as anaphylatoxins. These induce Degranulation which release of histamine and other pharmacologically active mediators. These induce smooth muscle contraction and increases in vascular permeability.
3. Lipid Inflammatory Mediators:
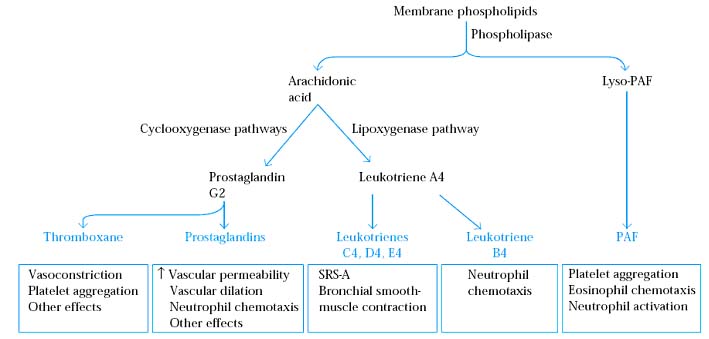
Lipid mediators include Platelet Activating Factor (PAF), Prostaglandins, thromboxanes and Leukotrienes. PAF causes platelet activation and has many inflammatory effects including eosinophil chemotaxis and the activation and Degranulation of neutrophils and eosinophils. Prostaglandins induce vascular permeability; vascular dilation and neutrophil chemotaxis. Thromboxanes induce vasoconstriction and platelet aggregation.
Leukotrienes include LTB4, LTC4, LTD4 and LTE4. Three of these namely LTC4, LTD4 and LTE4 together called as slow reacting substance of anaphylaxis (SRS – A). It induces smooth muscle contraction. These lipid inflammatory mediators are called as secondary mediators in allergic response.
4. Acute phase proteins (APP):
The local inflammation that develops at the site of infection induces the acute phase response. This generalized response is characterized by fever, changes in vascular permeability and changes in biosynthesis, metabolism and catabolism in many organs. These changes result in a raise in the concentration of certain proteins in the blood and a drop in the concentration of other proteins. These proteins, termed as acute phase proteins or acute phase reactants. These include C- reactive protein, fibrinogen and serum amyloid A protein. Hepatic synthesis of these molecules is upregulate by inflammatory cytokines, especially IL – 6 and TNF.
The major acute phase reactants in mammals are serum amyloid A and either C – reactive protein or serum amyloid P component (SAP) depending on the species. SAA and CRP are major acute phase reactants in humans. The concentration of CRP in the blood increases from a normal level of 1 mg / ml to as much as 1 mg / ml during the acute phase response. CRP and SAP are pentraxins, proteins with single or double ring like structures respectively, each ring consisting of five identical subunits forming a pentagon. Both CRP and SAP function in clearance of nuclear material released from killed microbes and killed host cells during inflammation by binding to DNA, chromatin and histones and in activation of the classical complement pathway by binding to C1q. CRP was originally named for its ability to bind the Capsular polysaccharide of the pneumococcus bacteria, which cause pneumonia. It can also bind t other bacteria; to parasites and to immune complexes, however, and it acts as an opsonin for their phagocytosis. It also enhances the killing activity of NK cells and macrophages.
In addition to the major acute phase reactants, other proteins are induced during the acute phase response. These include complement proteins, coagulation proteins, protease inhibitors, metal binding proteins, mannose binding protein and lipopolysaccharide binding proteins. Activation of the complement cascade at the sites of infection results in the killing of microbes and in the clearance of foreign and host cellular debris. The blood clotting cascade leads to decreased vascular permeability and thus controls the process of inflammation. Protease inhibitors that neutralize the lysosomal enzymes released by activated macrophages and neutrophils during phagocytosis. This prevents or reduces damage to host tissues. Metal binding proteins bind iron preventing its uptake by bacteria which require iron for growth. It also reacts with oxygen free radicals inactivating them and thereby limiting tissue damage. Mannose binding protein activates Lectin pathway through which it degrades some bacteria. MBP interact with lipopolysaccharides from gram negative bacteria. This complex binds to CD14, the lipopolysaccharide receptor on macrophages and greatly enhances macrophages activation compared to the binding of lipopolysaccharides alone.
TYPES OF INFLAMMATORY RESPONSE:
Inflammatory responses are of two type’s namely acute and chronic inflammatory response. Acute inflammatory response occurs immediately after the entry of antigen. It is mediated by APPs, C5a, C3a, C4a, MIF 1a and MIF 1b. Chronic inflammatory response develops during persistence of an antigen. Chronic inflammatory response mediated by Tc cells, NK cells and macrophages etc.
ANTI INFLAMMATORY AGENS:
These are three types namely
a) Agents preventing extravasation
b) Corticosteroids
c) Non steroidal anti-inflammatory drugs (NSAIDs)
a) Agents preventing extravasation:
Extravasation of leucocytes was prevented by blocking the activities of various adhesion molecules with antibodies. Example: Antibodies against LFA – 1 and ICAM – 1 used to block extravasation of leukocytes. Since, extravasation is an integral part of inflammation, impediating this inturn inhibits inflammatory response.
b) Corticosteroids:
The corticosteroids which are cholesterol derivatives include prednisone; prednisolone and methylprednisolone result in reduction in the numbers and activity of immune system cells. Corticosteroids treatment causes a decrease in the number of circulating lymphocytes as the result of either steroid induced lysis of lymphocytes or of alterations in lymphocyte circulation patterns. Like other steroid hormones, the corticosteroids are lipophilic and thus can cross the plasma membrane and bind to receptors in cytosol. The resulting receptor hormone complexes are subsequently transported to the nucleus where they bind to specific regulatory DNA sequences either up regulating or down regulating transcription. The corticosteroids have been shown to induce increased transcription of the NF Kb inhibitors. Binding of this inhibitor to NF Kb in the cytosol prevents the translocation of NF Kb into the nucleus and consequently prevents NF Kb activation of a number of genes including genes involved in T-cell activation and cytokine production.
Corticosteroids also reduce both the phagocytic and killing ability of macrophages and neutrophils. Corticosteroids also stabilize the lysosomal membrane, so that decreased levels of lysosomal enzymes are released at the site of Inflammation.
c) Non Steroidal Anti-Inflammatory Drugs (NSAIDs):
NSAIDs are the most frequently used medication for treating pain and inflammation. One mechanism by which threes drugs exert anti inflammatory effect is by inhibiting the cyclooxygenase pathway by which prostaglandins and thromboxanes are produced from arachidonic acid. The reduction in prostaglandins production limits the increase in vascular permeability and neutrophil chemotaxis in the inflammatory response. Aspirin is an example for NSAIDs.
FACTORS AFFECTING INNATE IMMUNITY:
Factors like age, hormonal influence and nutrition influence the level of innate immunity in an individual.
Age:
The two extremes of life i.e. infants and old, carry higher susceptibility to infectious diseases as compared to adults. The fetus in utero is normally protected from maternal infection by the placental barrier. But some pathogens cross this barrier causing overwhelming infections resulting in fetal death. Some such as rubella and cytomegalo viruses and Toxoplasma gondii, lead to congenital malformation. The heightened susceptibility of the fetus to infection is related to the immaturity of its immune apparatus. Old persons are highly susceptible to infections to the gradual waning of their immune responses.
Hormonal Influences:
Endocrine disorders such as diabetes mellitus, hypothyroidism and adrenal dysfunction are associated with an enhanced susceptibility to infections. The high incidence of staphylococcal sepsis in diabetes may be related to the increased level of carbohydrates in tissues. Corticosteroids exert an important influence on the responses to infection. They depress the host’s resistance by their anti-inflammatory and antiphagocytic effects and by the suppression of antibody formation and hypersensitivity.
Nutrition:
The interaction between malnutrition and immunity is complex but in general both humoral and cell mediated immune processes are reduced in malnutrition. Cell mediated immune responses such as the Manitou test become negative in severe protein deficiency as in kwashiorkor. Because of its wide prevalence, malnutrition may well be the commonest form of immunodeficiency.
ACQUIRED OR SPECIFIC OR ADAPTIVE IMMUNITY:
The form of immunity that is mediated by lymphocytes and stimulated by exposure to infectious agents is adaptive immunity. It reflects the presence of a functional immune system that is capable of specifically recognizing and selectively eliminating foreign microorganism and molecules. It is characterized by four characteristics namely
a. Antigenic specificity
b. Diversity
c. Immunologic memory
d. Self / Non-self recognition
a. Antigenic specificity:
A cardinal feature of the adaptive immunity, namely those immune responses are directed toward and able to distinguish between distinct antigens or small parts of macromolecular antigens. This fine specificity is attributed to lymphocyte antigen receptors that may bind to one molecule but not to another with only minor structural differences from the first. Antibodies can differentiate between two molecules that differ by only a single aminoacid.
b. Diversity:
Diversity is the fundamental property of the adaptive immunity and is the result of variability in the structure of the antigen binding sites of lymphocyte receptors for antigens. Example: Paratopes of antibodies and TCR of T-cells. Large number of lymphocytes with different antigenic specificities exists. Diversity allows adaptive immune system to specifically recognize billions of uniquely different structures on foreign antigens.
c. Immunologic memory:
The property of the adaptive immune system to respond more rapidly with greater magnitude and more effectively to a repeated exposure to an antigen, compared with the response to the first exposure. Immunologic memory mediated by memory cells. Memory cells are clonally expanded progeny of T and B cells formed during the primary response following initial exposure to antigen. Memory cells are more easily activated than naive lymphocytes and mediate secondary response on subsequent exposure to antigen. They survive in a functionally quiescent state for many years after the antigen eliminated. Due to this attribute, the immune system can confer life long immunity to many infectious agents.
d. Self and Non-self recognition:
It is also an important property of adaptive immunity. Due to this ability, immune system was able to distinguish self from nonself antigen and respond only to nonself molecules. If immune system responds to self antigen then it results in auto immune diseases. Production of immune cells only against non-self molecules was achieved by selection procedure like positive selection and negative selection during maturation process of lymphocytes in bone marrow and thymus.
Acquired immunity does not occur independently of innate immunity and vice versa. For example, the phagocytic cells crucial to nonspecific immune responses are intimately involved in activation of the specific immune response. Conversely, the soluble factors produced during a specific immune response, have been shown to augment the activity of these phagocytic cells.
Acquired immunity on the basis of component involved in immunity classified in to two types namely Humoral immunity and Cell mediated immunity.
HUMORAL IMMUNITY:
The term humoral is derived from the Latin humour meaning “body fluid”, thus humoral immunity refers to immunity that can be conferred on a nonimmune individual by administration of serum antibodies from an immune individual. Humoral immunity is mediated by molecules in blood and mucosal secretions called antibodies that are produced by cells called B lymphocytes.
Antibodies recognize microbial antigens, neutralize infectivity of the microbes and target microbes for elimination by various effector mechanisms. Antibodies themselves are specialized and different types of antibodies may activate different mechanisms, for example, some types of antibodies promote phagocytosis and others triggers the release of inflammatory mediators from leukocytes such as mast cells. Binding of antibody to antigen on a microorganism also can activate the complement system, resulting in lysis of the foreign organism.
Humoral immunity is the principal defense mechanism against extracellular microbes and their toxins because secreted antibodies can bind to these microbes and toxins and assist in their elimination.
CELL MEDIATED IMMUNITY:
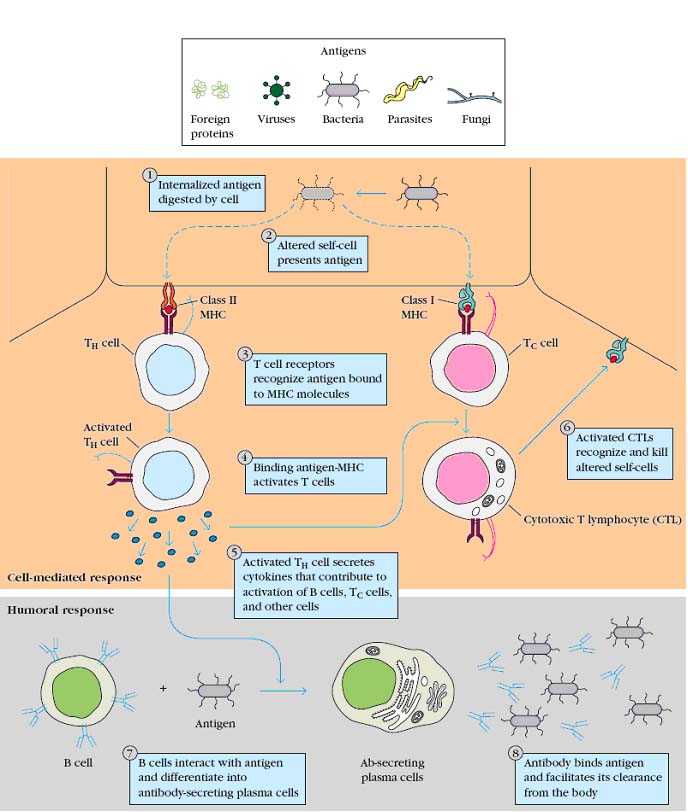
Cell Mediated immunity also called as cellular immunity, is mediated by T lymphocytes or T cells. There are two types of T-cells mainly TH and TC, which differ by their surface marker CD4 and CD8 respectively. Effector T cells generated in response to antigen are responsible for cell mediated immunity. Both activated TH cells and TC cells serve as effector cells in CMI. Cytokines secreted by TH cells can activate various phagocytic cells, enabling them to phagocytose and kill microorganisms more effectively. Cytotoxic T lymphocytes (CTL) participate in CMI by killing altered self cells. They also play an important role in the killing of virus infected cells and tumor cells.
Intracellular microbes, such as viruses and some bacteria, survive and proliferate inside phagocytes and other host cells, where they are inaccessible to circulating antibodies. Defense against such infections is a function of cell mediated immunity.
Generation of the Humoral Response:
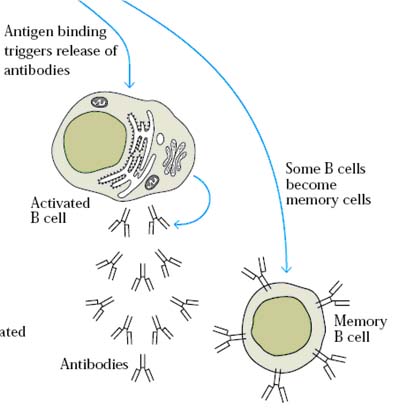
Mature antigen committed B lymphocytes are comes out from the bone marrow to circulate in the blood or lymph or to reside in various lymphoid organs. Interaction of the mature B-cell with antigen triggers its activation and further proliferation and differentiation. This process begins when antigen cross links membrane bound antibody molecules on B-cell. Some of the bound antigen is internalized by receptor mediated endocytosis. After processing the antigen, the B cell presents the resulting antigenic peptides together with class II MHC molecules on its membrane. A TH cell specific for the presented Ag-MHC complex then binds to the complex. As a result of this interaction, the TH cell secretes a number of cytokines that stimulate various stages of B-cell divisions and differentiation.
The activated B-cell undergo a series of cell division over approximately a 5 day period differentiating into a population of both antibody secreting plasma cells and memory cells.
Generation of Cell Mediated Response:
The cell mediated response is generated by various subpopulations of T lymphocytes. After a TH cells exposed to antigen through APCs, it’s activated. Cytokines secreted by activated TH cells help to activate various T effector cells responsible for cell mediated response. For example, after a Tc cell binds to processed antigen associated with MHC-I molecules on the membrane of an altered self cell, IL-2 secreted by TH cells stimulates and proliferation and differentiation of the Tc cells.
This process generates cytotoxic T lymphocytes, which mediates membrane damage to the altered self cell leading to cell lysis as well as populations of memory TH cells and Tc cells. The cytokines released from TH cells also regulate the proliferation and differentiation of a number of nonspecific effector cells that play various roles in cell mediated immune responses. These nonspecific effector cells do not possess the immunologic attributes of specificity and memory. Instead, their activity is regulated by cytokines secreted by antigen specific TH cells. Among the nonspecific effector cells involved in cell mediated immunity are NK cells and activated macrophages.
IMMUNE RESPONSE:
A Collective and coordinated response to the introduction of foreign substances in an individual, mediated by the cells and molecules of the immune system is referred as immune response. Immune response (Ir) genes originally defined as genes in inbred strains of rodents that were inherited in a dominant Mendelian manner and that controlled the ability of the animals to make antibodies against simple synthetic polypeptides. Ir genes are polymorphic MHC genes that encode peptide binding molecules required for the activation of T lymphocytes and are therefore also required for TH cells dependent B-cell response to protein antigen.
Antigens are captured from their site of entry by dendritic cells (Antigen Presenting cells) and transported into lymph nodes via lymphatic circulation. In lymph nodes, naïve lymphocytes activated, proliferated and differentiated into effector and memory lymphocytes. Effector and memory lymphocytes developed in the nodes, enter the circulation from which they may migrate to peripheral tissues. Effector T-cells and antibodies produced from plasma cells eliminate antigens whereas memory lymphocytes take up residence in normal tissues in preparation for next or future infection.
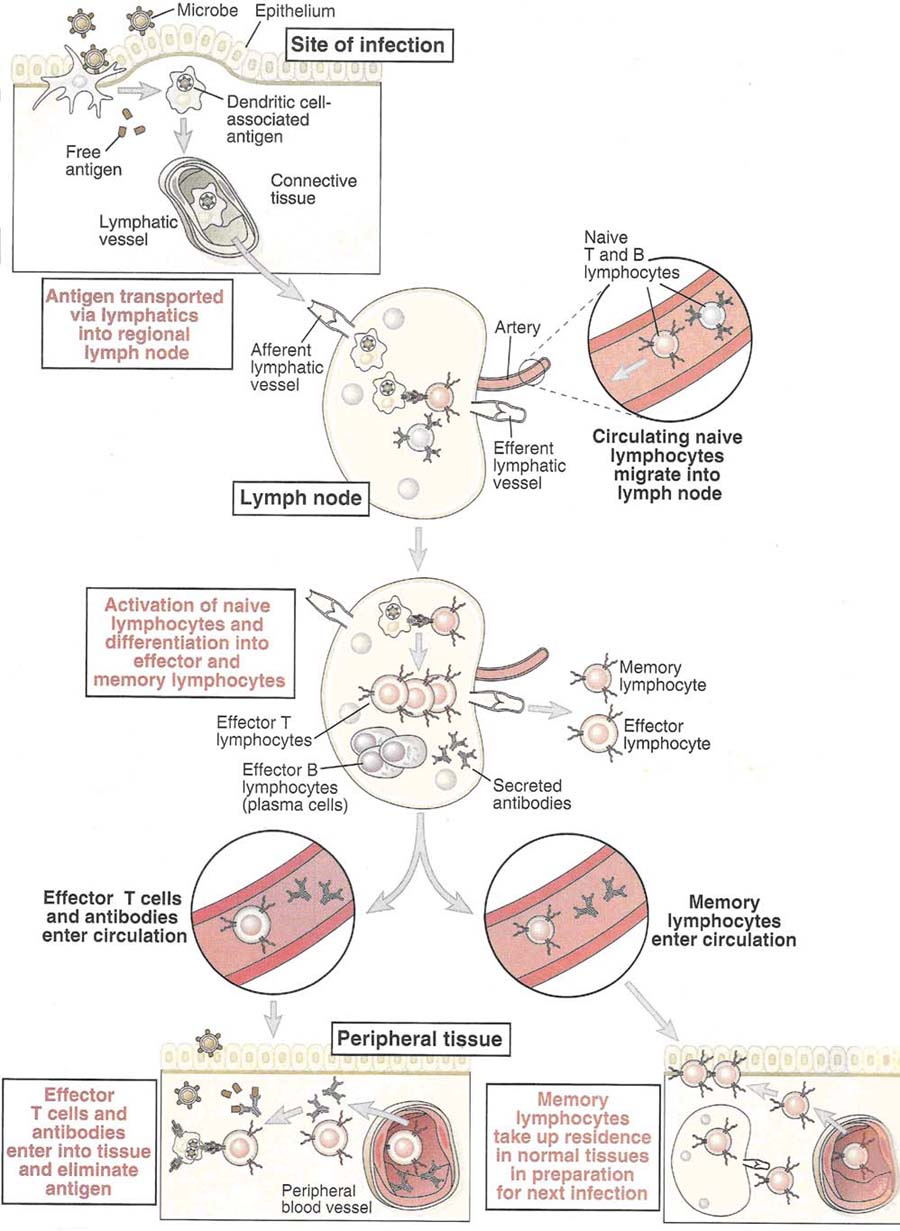
Immune response is of two types namely Primary Immune Response and Secondary Immune Response.
Primary Immune Response:
An adaptive immune response that occurs upon the first exposure of native lymphocytes with a foreign antigen is known as primary immune response. Primary responses are characterized by relatively slow kinetics and small magnitude compared with the response after a second or subsequent exposure. It has lag phase, log phase, effector phase and decline phase.
Secondary Immune Response:
An adaptive immune response that occurs upon the second or subsequent encounter of primed lymphocytes with a given antigen is known as secondary immune response. A secondary response is characterized by more rapid kinetics and greater magnitude relative to the primary immune response which occurs on first encounter. It has log phase, effector phase and decline phase. Absence of lag phase is significant in secondary immune response. Secondary immune response proves that immune system remembers the first encounter and is said to have memory. This can be illustrated with the following example: Most of us were first exposed to the varicella-zoaster virus during childhood and came down with chickenpox. After this exposure we became immune to chickenpox, so subsequent exposure to the varicella-zoaster virus no longer results in illness. This immunologic memory or maintenance of the acquired immunity is often life long. The memory is specific for the original invader, in this case the varicella-zoaster virus and does not extend to unrelated invaders such as other viruses.
Immune Response and Humoral immunity:
In the humoral branch of the immune system, antigen induces clonal proliferation of B lymphocytes into plasma cell and memory B cells. The initial phase of primary response has a lag of 5 – 7 days before antibody levels to start to rise. This lag time is the time required for activation of naïve B-cells by antigen and TH cells and in log phase, subsequent proliferation and differentiation of the activated B cells into antibody secreting plasma cells and memory B cells occurs. Antibody levels peak in the primary immune response at about day 14 and then begin to drop off as the plasma cells begin to die.
In the secondary immune response, the lag is much shorter or even absent (1 – 2 days) and antibody levels are much higher and are sustained for much longer time. The secondary immune response reflects the response of the clonally expanded population of memory B cells. These memory B cells respond to antigen more rapidly than naïve B cells. In addition, because there are many more memory cells than naïve B cells, larger number of plasma cells are generated during the secondary immune response and antibody levels are consequently 100 fold or 1000 fold higher.
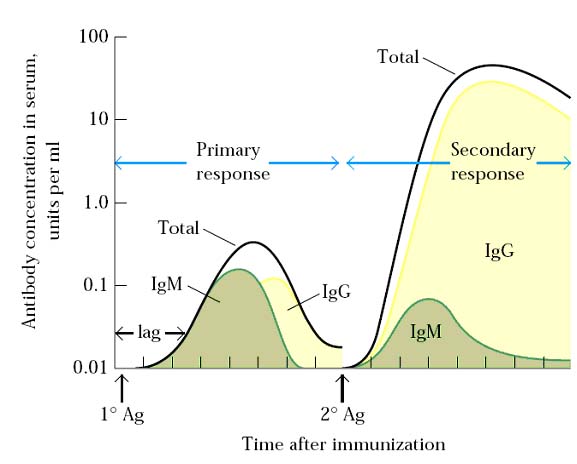
|
Features |
Primary Immune Response |
Secondary Immune Response |
|
Time Lag after Immunization |
Usually 5 – 7 days |
Usually 1 – 2 days |
|
Peak Response |
Smaller |
Larger |
|
Antibody Isotype |
Usually Ig M > Ig G |
Increased Ig G, Ig A or Ig E |
|
Antibody Affinity |
Lower average affinity |
Higher average affinity |
|
Induced by |
All immunogens |
Only protein antigens |
|
Required Immunization |
High dose, Adjuvant needed |
Low dose, Adjuvant not necessary |
Immune Response and Cell mediated immunity:
In the cell mediated branch, the recognition of an antigen – MHC complex by specific mature T lymphocytes induces clonal proliferation into various T cells with effector functions and into memory T cells. When skin from a strain C mouse is grafted onto a strain A mouse, a primary response develops and the graft is rejected in about 10 – 14 days. If strain C is grafted second time or subsequent grafting onto the same mouse, it is rejected much more vigorously and rapidly than the first graft (5 – 7 days). If a primary graft from strain B mouse is grafted onto a strain A mouse together with the graft from strain C, the response to strain B is a typical primary response. That is, the graft rejection is a specific immune response. The mouse shows secondary immune response to graft C. The increased speed of rejection of graft C reflects the presence of clonally expanded population of memory TH cells and TC cells to the antigen of the foreign graft. This expanded memory population will generate increased numbers of effector cells, resulting in faster graft rejection.
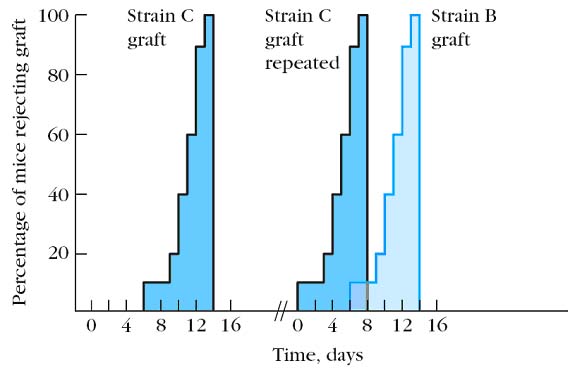
MEASUREMENT OF IMMUNITY:
The truly valid measurement of immunity is to test the resistance of an individual to a challenge by the pathogen. This is, however, not applicable since the challenge itself alters the state of immunity. It is, therefore, not possible to measure accurately the level of immunity in an individual. Estimates of immunity are generally made by statistical methods using large numbers of individuals.
A simple method of testing immunity is to relate its level to some convenient indicator, such as demonstration of the specific antibody. This is, however, not always reliable as the immune response to a pathogen consists of the formation of antibodies to several antigens present in it. Such antibodies may be demonstrated by a variety of techniques such as agglutination, precipitation, complement fixation, hemagglutination inhibition and neutralization. In the absence of exact information as to which of the antigens of the pathogen constitutes the ‘protective antigen’, serological attempts to measure immunity are at best but approximations. In some instances, as in diphtheria where pathogenesis is due to a well defined antigen i.e. diphtheria toxin, the level of immunity can be assayed by in vitro or in vivo i.e. shick test methods. Where protection is associated with cell mediated immunity, skin tests for delayed hypersensitivity and in vitro tests for CMI afford an indication of immunity.