The Hexokinase reaction involves nucleophilic attack of the C6 hydroxyl oxygen of glucose on the phosphorous of the terminal phosphate of ATP.
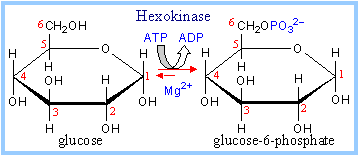
ATP binds to the enzyme as a complex with Mg++.
The Glycolysis pathway of Glycolysis take place in the cytosol of cells. Glucose enters the Glycolysis pathway by conversion to glucose-6-phosphate. Initially, there is energy input corresponding to cleavage of two ~P bonds of ATP. This pathway otherwise known as Embden-Meyerhof-Parnas pathway or EM pathway.
1. Hexokinase Reaction:
Hexokinase catalyzes the formation of glucose – 6 – phosphate from glucose and ATP.
glucose + ATP à glucose-6-phosphate + ADP
|
The Hexokinase reaction involves nucleophilic attack of the C6 hydroxyl oxygen of glucose on the phosphorous of the terminal phosphate of ATP.
ATP binds to the enzyme as a complex with Mg++. |
|
|
The positively charged Mg++ interacts with negatively charged phosphate oxygen atoms of ATP, providing charge compensation and promoting a favorable conformation of ATP at the active site. The reaction catalyzed by Hexokinase is highly spontaneous. A phosphoanhydride bond of ATP (~P) is cleaved. The phosphate ester formed in glucose-6-phosphate has a lower DG of hydrolysis. |
|
|
Induced fit: Glucose binding to Hexokinase stabilizes a conformation in which
It is a common motif for an enzyme active site to be located at an interface between protein domains that are connected by a flexible hinge region. The structural flexibility allows access to the active site, while permitting precise positioning of active site residues, and in some cases exclusion of water, as substrate binding promotes a particular conformation.
|
|
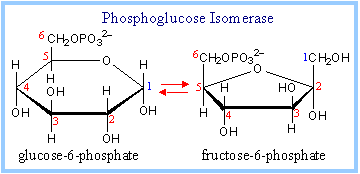
2. Phosphoglucose Isomerase Reaction:
It catalyzes the conversion of glucose – 6 – phosphate to fructose – 6 – phosphate.
glucose-6-phosphate (aldose) ßà fructose-6-phosphate (ketose)
|
The Phosphoglucose Isomerase mechanism involves acid/base catalysis, with ring opening, isomerization via an enediolate intermediate, and then ring closure . |
|
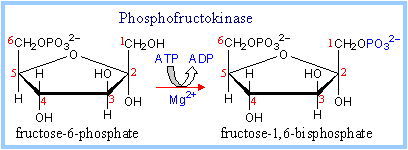
3. Phosphofructokinase Reaction :
It catalyzes the formation of fructose 1,6 bisphosphate from fructose-6-phosphate and ATP.
fructose-6-phosphate + ATP à fructose-1,6-bisphosphate + ADP
|
This highly spontaneous reaction has a mechanism similar to that of Hexokinase. The Phosphofructokinase reaction is the rate-limiting step of Glycolysis. The enzyme is highly regulated, as will be discussed later. |
|
4. Aldolase Reaction:
It catalyzes degrades fructose 1,6 bisphosphate to dihydroxyacetone phosphate and glyceraldehydes – 3- phosphate.
fructose-1,6-bisphosphate ßà dihydroxyacetone phosphate + glyceraldehyde-3-phosphate.
|
The Aldolase reaction is an aldol cleavage, the reverse of an aldol condensation. Note that carbon atoms are renumbered in reaction products.
|
|
|
|
A lysine residue at the active site of the Aldolase enzyme functions in catalysis. The keto group of fructose-1,6-bisP reacts with the e-amino group of the active site lysine, to form a protonated Schiff base intermediate.
Cleavage of the bond between C3 and C4 follows and results in the corresponding products. |
|
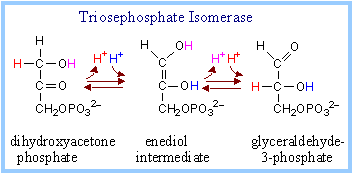
5. Triose Phosphate Isomerase (TIM) :
It catalyzes the interconversion of dihydroxy acetone phosphate to glyceraldehydes – 3- phosphate.
dihydroxyacetone phosphate (ketose) ßà glyceraldehyde-3-phosphate (aldose)
Glycolysis continued from glyceraldehydes – 3- phosphate. The equilibrium constant (Keq) for the TIM reaction favors dihydroxyacetone phosphate, but removal of glyceraldehyde-3-phosphate by a subsequent spontaneous reaction allows throughput.
|
The ketose/aldose conversion of TIM involves acid/base catalysis, and is thought to proceed via an enediol intermediate, as with Phosphoglucose Isomerase, Active site Glu and His residues are thought to extract and donate protons during catalysis. |
|
|
2-Phosphoglycolate is an example of a transition state analog that binds tightly at the active site of Triose Phosphate Isomerase. This inhibitor of catalysis by TIM is similar in structure to the proposed enediolate intermediate.
TIM is considered a "perfect enzyme", because the reaction rate is limited only by the rate at which substrate collides with the enzyme. |
|
|
|
|
|
|
|
6. Glyceraldehyde-3-phosphate Dehydrogenase :
It catalyzes formation of 1,3 bisphosphoglycerate from glyceraldehydes – 3- phosphate and inorganic phosphate. NAD+ also converted into NADH / H+.
glyceraldehyde-3-phosphate + NAD+ + Pi ßà 1,3,bisphosphoglycerate + NADH + H+
|
Exergonic oxidation of the aldehyde in glyceraldehyde-3-phosphate, to a carboxylic acid, drives formation of an acyl phosphate, a "high energy" bond (~P), in 1,3-bisphosphoglycerate. This is the only step in Glycolysis in which NAD+ is reduced to NADH.
|
|
|
A cysteine thiol at the active site of Glyceraldehyde-3-phosphate Dehydrogenase has a role in catalysis.
The aldehyde of glyceraldehyde-3-phosphate reacts with the active site cysteine thiol to form a thiohemiacetal intermediate. Oxidation to a carboxylic acid (in a "high energy" thioester) occurs, as NAD+ is reduced to NADH. The "high energy" acyl thioester is attacked by Pi to yield the acyl phosphate (~P) product. |
|
7. Phosphoglycerate Kinase :
It catalyzes the formation of 3 – phosphoglycerate from 1,3 – bisphosphoglycerate and ADP.
1,3-bisphosphoglycerate + ADP ßà 3-phosphoglycerate + ATP
|
This transfer of phosphate to ADP, from the carboxyl group on 1,3-bisphosphoglycerate, is reversible (low DG), since one ~P bond is cleaved and another is synthesized. The enzyme undergoes a substrate-induced conformational change similar to that of Hexokinase.
|
|
8. Phosphoglycerate Mutase :
It catalyzes the isomerization reaction in which 3-phosphoglycerate is converted into 2-phosphoglycerate.
3-phosphoglycerate ßà 2-phosphoglycerate
|
Phosphate is shifted from the hydroxyl on C3 of 3-phosphoglycerate to the hydroxyl on C2.
|
|
|
|
An active site histidine side-chain participates in phosphate transfer, by donating and accepting the phosphate. The process involves a 2,3-bisphosphate intermediate. |
9. Enolase Reaction:
It catalyzes conversion of 2-phosphoglycerate to phosphoenolpyruvate.
2-phosphoglycerate ßà phosphoenolpyruvate + H2O
|
This dehydration reaction is Mg++-dependent. 2 Mg++ ions interact with oxygen atoms of the substrate carboxyl group at the active site. The Mg++ ions help to stabilize the enolate anion intermediate that forms when a lysine side-chain amino group extracts a proton from carbon #2.
|
|
10. Pyruvate Kinase Reaction :
It catalyzes formation of pyruvate and ATP from Phosphoenolpyruvate and ADP.
phosphoenolpyruvate + ADP à pyruvate + ATP
|
This transfer of phosphate from PEP to ADP is spontaneous. PEP has a larger DG of phosphate hydrolysis than ATP, because removal of phosphate from PEP yields an unstable enol, that spontaneously converts to the keto form of pyruvate. Required inorganic cations K+ and Mg++ bind to anionic residues at the active site of Pyruvate Kinase.
|
|
|
Summary of Glycolysis: The pathway continues from glyceraldehyde-3-phosphate. Recall that there are two glyceraldehyde-3-phosphate per glucose metabolized. |
|
Balance sheet for high energy bonds of ATP:
|
|
Glycolysis Pathway (omitting H+):
glucose + 2 NAD+ + 2 ADP + 2 Pi à 2 pyruvate + 2 NADH + 2 ATP
Fate of Pyruvate after glycolysis :
In aerobic organisms, pyruvate produced in Glycolysis is oxidized to CO2 via Krebs Cycle, and the NADH produced in Glycolysis and Krebs Cycle is reoxidized via the respiratory chain, with production of much additional ATP.
Anaerobic organisms lack a respiratory chain. They must reoxidize NADH produced in Glycolysis through some other reaction, because NAD+ is needed for the Glyceraldehyde-3-phosphate Dehydrogenase reaction. Usually NADH is reoxidized as pyruvate is converted to a more reduced compound.
The complete pathway, including Glycolysis and the re-oxidation of NADH, is called fermentation.
|
For example, Lactate Dehydrogenase catalyzes reduction of the keto group in pyruvate to a hydroxyl, yielding lactate, as NADH is oxidized to NAD+.
Lactate, in addition to being an end-product of fermentation, serves as a mobile form of nutrient energy, and possibly as a signal molecule in mammalian organisms. Cell membranes contain carrier proteins that facilitate transport of lactate.
·
Lactate serves as a fuel source for cardiac muscle as well as
brain neurons. |
|
|
Some anaerobic organisms metabolize pyruvate to ethanol, which is excreted as a waste product. NADH is converted to NAD+ in the reaction catalyzed by Alcohol Dehydrogenase. Thiamine pyrophosphate, the cofactor for Alcohol Dehydrogenase, is discussed elsewhere.
|
|
Fermentation Pathway, from glucose to lactate (omitting H+):
glucose + 2 ADP + 2 Pi à 2 lactate + 2 ATP
Anaerobic catabolism of glucose yields only 2
“high energy” bonds of ATP.
Regulation of Glycolysis:
|
Glycolysis Enzyme * |
DGo' (kJ/mol) |
DG (kJ/mol) |
|
Hexokinase |
-20.9 |
-27.2 |
|
Phosphoglucose Isomerase |
+2.2 |
-1.4 |
|
Phosphofructokinase |
-17.2 |
-25.9 |
|
Aldolase |
+22.8 |
-5.9 |
|
Triosephosphate Isomerase |
+7.9 |
negative |
|
Glyceraldehyde-3-phosphate Dehydrogenase, & Phosphoglycerate Kinase |
-16.7 |
-1.1 |
|
Phosphoglycerate Mutase |
+4.7 |
-0.6 |
|
Enolase |
-3.2 |
-2.4 |
|
Pyruvate Kinase |
-23.0 |
-13.9 |
Flux through the Glycolysis pathway is regulated by control of the 3 enzymes that catalyze highly spontaneous reactions: Hexokinase, Phosphofructokinase, & Pyruvate Kinase.
Hexokinase, the first step in the Glycolysis pathway, is inhibited by its product glucose-6-phosphate:
Cells trap glucose by phosphorylating it, preventing exit on glucose carriers. Product inhibition of Hexokinase ensures that cells will not continue to accumulate glucose from the blood, if [glucose-6-phosphate] within the cell is ample.
Glucokinase is a variant of Hexokinase found in liver.
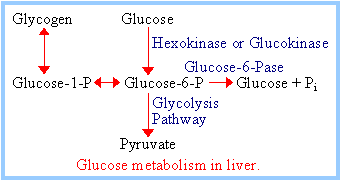
|
Glucokinase, with its high KM for glucose, allows the liver to store glucose as glycogen in the fed state when blood [glucose] is high. The liver enzyme Glucose-6-phosphatase catalyzes hydrolytic release of Pi from glucose-6-phosphate. Thus glucose is released from the liver to the blood as needed to maintain blood [glucose]. The enzymes Glucokinase and Glucose-6-phosphatase, both found in liver but not in most other body cells, allow the liver to control blood [glucose]. |
Pyruvate Kinase, the last step of the Glycolysis pathway, is controlled in liver partly by modulation of the amount of enzyme.
|
Phosphofructokinase is usually the rate-limiting step of the Glycolysis
pathway. Phosphofructokinase catalyzes: fructose-6-phosphate + ATP à fructose-1,6-bisphosphate + ADP Phosphofructokinase (PFK) is allosterically inhibited by ATP. At low concentration, the substrate ATP binds only at the active site. At high concentration, ATP binds also at a lower-affinity regulatory site, promoting the tense conformation. The tense conformation of Phosphofructokinase has a lower affinity for its other substrate, fructose-6-phosphate. Sigmoidal dependence of reaction rate on [fructose-6-phosphate] is observed at high [ATP], as depicted at right. |
AMP, which is present at significant levels only when there is extensive ATP hydrolysis, antagonizes the effect of high [ATP].
Inhibition of Phosphofructokinase, the rate-limiting step of Glycolysis, when [ATP] is high, prevents breakdown of glucose, in a pathway whose main role is to make ATP. It is more useful to the cell to store glucose as glycogen when ATP is plentiful.
Usually, bacterial fermentations are distinguished by their end products into the following groups.
1. Homolactic Fermentation. Lactic acid is the sole end product. Pathway of the homolactic acid bacteria (Lactobacillus and most streptococci). The bacteria are used to ferment milk and milk products in the manufacture of yogurt, buttermilk, sour cream, cottage cheese, cheddar cheese, and most fermented dairy products.
2. Mixed Acid Fermentations. Mainly the pathway of the Enterobacteriaceae. End products are a mixture of lactate, acetate, formate, succinate and ethanol, with the possibility of gas formation (CO2 and H2) if the bacterium possesses the enzyme formate dehydrogenase, which cleaves formate to the gases.
2a. Butanediol Fermentation. Forms mixed acids and gases as above, but, in addition, 2,3 butanediol from the condensation of 2 pyruvate. The use of the pathway decreases acid formation (butanediol is neutral) and causes the formation of a distinctive intermediate, acetoin. Water microbiologists have specific tests to detect low acid and acetoin in order to distinguish non fecal enteric bacteria (butanediol formers, such as Klebsiella and Enterobacter) from fecal enterics (mixed acid fermenters, such as E. coli, Salmonella and Shigella).
3. Butyric acid fermentations, as well as the butanol-acetone fermentation (below), are run by the clostridia, the masters of fermentation. In addition to butyric acid, these clostridia form acetate, CO2 and H2 from the fermentation of sugars. Small amounts of ethanol and isopropanol may also be formed.
3a. Butanol-acetone fermentation. Butanol and acetone were discovered as the main end products of fermentation by Clostridium acetobutylicum during the World War I. This discovery solved a critical problem of explosives manufacture (acetone is required in the manufacture gunpowder) and is said to have affected the outcome of the war. Acetone was distilled from the fermentation liquor of Clostridium acetobutylicum, which worked out pretty good if you were on our side, because organic chemists hadn't figured out how to synthesize it chemically. You can't run a war without gunpowder.
4. Propionic acid fermentation. This is a bizarre fermentation carried out by the propionic acid bacteria which include corynebacteria, Propionibacterium and Bifidobacterium. Although sugars can be fermented straight through to propionate, propionic acid bacteria will ferment lactate (the end product of lactic acid fermentation) to acetate, CO2 and propionate. The formation of propionate is a complex and indirect process involving 5 or 6 reactions. Overall, 3 moles of lactate are converted to 2 moles of propionate + 1 mole of acetate + 1mole of CO2, and 1 mole of ATP is squeezed out in the process. The propionic acid bacteria are used in the manufacture of Swiss cheese, which is distinguished by the distinct flavor of propionate and acetate, and holes caused by entrapment of CO2.