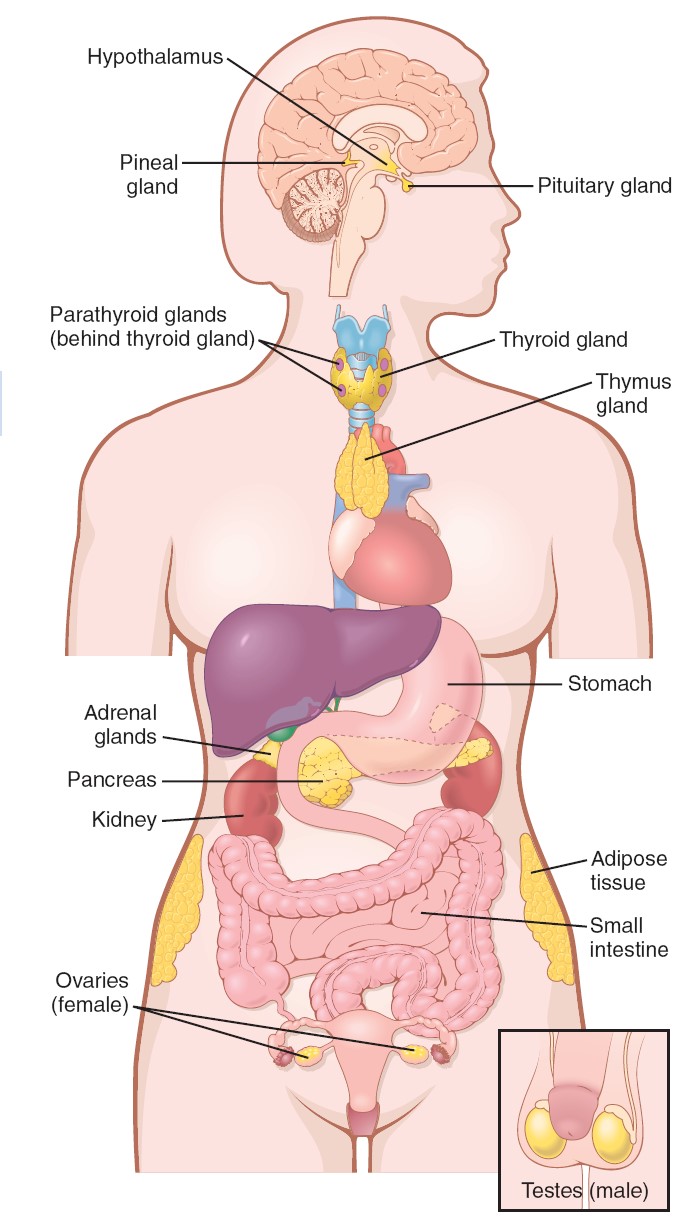
ENDOCRINE SYSTEM
Hormones are regulatory molecules secreted into the blood by endocrine glands.
Chemical categories of hormones include steroids, amines, polypeptides, and
glycoproteins. Interactions between the various hormones produce effects that
may be synergistic, permissive, or antagonistic.
Endocrine glands
lack the ducts that are present in exocrine glands. The endocrine glands secrete
their products, which are biologically active molecules called hormones,
into the blood. The blood carries the hormones to target cells that
contain specific receptor proteins for the hormones, and which therefore
can respond in a specific fashion to them. Many endocrine glands are organs
whose primary functions are the production and secretion of hormones. The
pancreas functions as both an exocrine and an endocrine gland; the endocrine
portion of the pancreas is composed of clusters of cells called the pancreatic
islets (islets of Langerhans). The concept of the endocrine system,
however, must be extended beyond these organs, because many other organs in the
body secrete hormones. These organs may be categorized as endocrine glands even
though they serve other functions as well. It is appropriate, then, that a
partial list of the endocrine glands should include the heart, liver, adipose
tissue, and kidneys. Some specialized neurons, particularly in the hypothalamus,
secrete chemical messengers into the blood rather than into a narrow synaptic
cleft. In these cases, the chemical that the neurons secrete is sometimes called
a neurohormone.
In addition, a number of chemicals—norepinephrine, for example—are secreted both
as a neurotransmitter and a hormone. Thus, a sharp distinction between the
nervous system and the endocrine system cannot always be drawn on the basis of
the chemicals they release. Hormones affect the metabolism of their target
organs and, by this means, help regulate total body metabolism, growth, and
reproduction.
|
Endocrine Gland |
Major
Hormones |
Primary Target Organs |
Primary Effects |
|
Adipose tissue |
Leptin
|
Hypothalamus
|
Suppresses appetite |
|
Adrenal cortex |
Glucocorticoids Aldosterone |
Liver and muscles Kidneys |
Glucocorticoids influence glucose metabolism; aldosterone promotes Na+
retention, K+ excretion |
|
Adrenal medulla |
Epinephrine
|
Heart, bronchioles, and blood vessels |
Causes adrenergic stimulation |
|
Heart
|
Atrial natriuretic hormone |
Kidneys
|
Promotes excretion of Na+ in the urine |
|
Hypothalamus
|
Releasing and inhibiting hormones |
Anterior pituitary |
Regulates secretion of anterior pituitary hormones |
|
Small intestine |
Secretin and cholecystokinin |
Stomach, liver, and pancreas |
Inhibits gastric motility and stimulates bile and pancreatic juice
secretion |
|
Islets of Langerhans (pancreas) |
Insulin Glucagon |
Many organs Liver and adipose tissue |
Insulin promotes cellular uptake of glucose and formation of glycogen
and fat; glucagon stimulates hydrolysis of glycogen and fat |
|
Kidneys
|
Erythropoietin
|
Bone marrow |
Stimulates red blood cell production |
|
Liver
|
Somatomedins
|
Cartilage
|
Stimulates cell division and growth |
|
Ovaries
|
Estradiol-17β and progesterone |
Female reproductive tract and mammary glands |
Maintains structure of reproductive tract and promotes secondary sex
characteristics |
|
Parathyroid glands |
Parathyroid hormone |
Bone, small intestine, and kidneys |
Increases Ca2+ concentration in blood |
|
Pineal gland |
Melatonin
|
Hypothalamus and anterior pituitary |
Affects secretion of gonadotrophic hormones |
|
Pituitary, anterior |
Trophic hormones |
Endocrine glands and other organs |
Stimulates growth and development of target organs; stimulates secretion
of other hormones |
|
Pituitary, posterior |
Antidiuretic hormone Oxytocin |
Kidneys and blood vessels Uterus and mammary glands |
Antidiuretic hormone promotes water retention and vasoconstriction;
oxytocin stimulates contraction of uterus and mammary secretory units |
|
Skin
|
1,25-Dihydroxyvitamin D3 |
Small intestine |
Stimulates absorption of Ca2+ |
|
Stomach
|
Gastrin
|
Stomach
|
Stimulates acid secretion |
|
Testes
|
Testosterone
|
Prostate, seminal vesicles, and other |
Stimulates secondary sexual development |
|
|
|
organs
|
|
|
Thymus
|
Thymopoietin
|
Lymph nodes |
Stimulates white blood cell production |
|
Thyroid gland |
Thyroxine (T4) and triiodothyronine ( T3); calcitonin |
Most organs |
Thyroxine and triiodothyronine promote growth and development and
stimulate basal rate of cell respiration (basal metabolic rate or BMR);
calcitonin may participate in the regulation of blood Ca2+ levels |
Chemical Classification of Hormones
Hormones secreted by different endocrine glands vary widely in chemical
structure. All hormones, however, can be divided into a few chemical classes.
1. Amines.
These are hormones derived from the amino acids tyrosine and
tryptophan. They include the hormones secreted by the adrenal medulla, thyroid,
and pineal glands.
2. Polypeptides and proteins.
Proteins are large
polypeptides, so the distinction between the two categories is somewhat
arbitrary. Antidiuretic hormone is a polypeptide with eight amino acids, too
small to accurately be called a protein. If a polypeptide chain is larger than
about 100 amino acids, such as growth hormone with 191 amino acids, it can be
called a protein. Insulin blurs the two categories, because it is composed of
two polypeptide chains derived from a single, larger molecule.
3. Glycoproteins.
These molecules consist of a protein bound to one or more
carbohydrate groups. Examples are follicle stimulating hormone (FSH) and
luteinizing hormone (LH).
4. Steroids.
Steroid hormones are derived from cholesterol after an enzyme
cleaves off the side chain attached to the five-carbon “D” ring. Steroid
hormones include testosterone, estradiol, progesterone, and cortisol. In terms
of their actions in target cells, hormone molecules can be divided into those
that are polar, and therefore water-soluble, and those that are nonpolar, and
thus insoluble in water.
Because the nonpolar hormones are soluble in lipids, they are often referred to
as lipophilic hormones. Unlike the polar hormones, which cannot pass
through plasma membranes, lipophilic hormones can gain entry into their target
cells. These lipophilic hormones include the steroid hormones and thyroid
hormones. Steroid hormones are
secreted by only two endocrine glands: the adrenal cortex and the gonads. The
gonads secrete sex steroids; the adrenal cortex secretes
corticosteroids (including cortisol and aldosterone) and small amounts of
sex steroids.
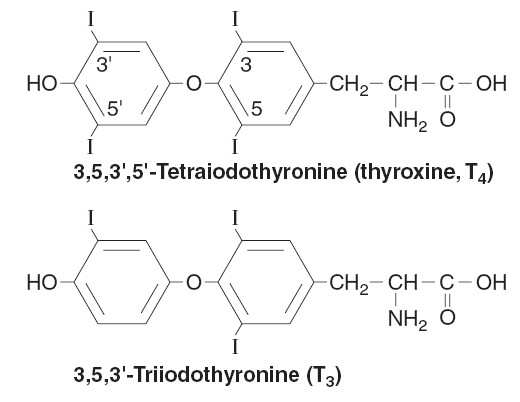
The major thyroid hormones are composed of two derivatives of the amino acid
tyrosine bonded together. When the hormone contains 4 iodine atoms, it is called
tetraiodothyronine (T4), or thyroxine. When it contains 3
atoms of iodine, it is called triiodothyronine (T3). Although
these hormones are not steroids, they are like steroids in that they are
relatively small, nonpolar molecules. Steroid and thyroid hormones are active
when taken orally (as a pill). Sex steroids are the active agents in
contraceptive pills, and thyroid hormone pills are taken by people whose thyroid
is deficient (who are hypothyroid). By contrast, polypeptide and glycoprotein
hormones cannot be taken orally because they would be digested into inactive
fragments before being absorbed into the blood. Thus, insulin-dependent
diabetics must inject themselves with this hormone. Polar, water-soluble
hormones include polypeptides, glycoproteins, and the catecholamine
hormones secreted by the adrenal medulla, epinephrine and norepinephrine. These
hormones are derived from the amino acid tyrosine. Thus, like the polypeptide
and glycoprotein hormones, the catecholamines are too polar to pass through the
phospholipid portion of the plasma membrane. The hormone secreted by the pineal
gland, melatonin, is different; derived from the nonpolar amino acid tryptophan,
melatonin pills can be effective because (like steroids and thyroxine) this
hormone can pass through plasma membranes. Melatonin, however, also has some
similarities to the polar hormones in terms of its effects on cells.
Prohormones and Prehormones
Hormone molecules that affect the metabolism of target cells are often derived
from less active “parent,” or precursor, molecules. In the case of
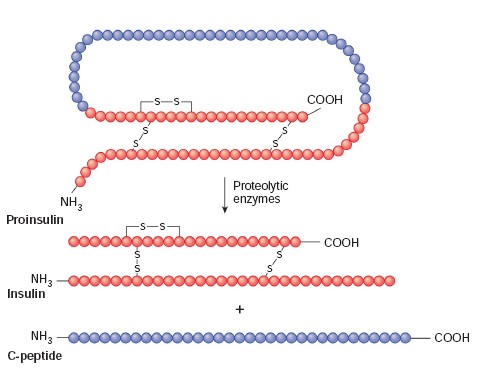
polypeptide hormones, the precursor may be a longer chained prohormone
that is cut and spliced together to make the hormone. Insulin, for example, is
produced from proinsulin within the beta cells of the islets of
Langerhans of the pancreas. In some cases, the prohormone itself is derived from
an even larger precursor molecule; in the case of insulin, this molecule is
called preproinsulin. The term prehormone is sometimes used to
indicate such precursors of prohormones. In some cases, the molecule secreted by
the endocrine gland (and considered to be the hormone of that gland) is actually
inactive in the target cells. In order to become active, the target cells must
modify the chemical structure of the secreted hormone. Thyroxine (T4), for
example, must be
changed into T 3 within the target cells in order to affect the
metabolism of these cells. Similarly, testosterone (secreted by the testes) and
vitamin D 3 (secreted by the skin) are converted into more active molecules
within their target cells. In this text, the term prehormone will be used
to designate those molecules secreted by endocrine glands that are inactive
until changed by their target cells.
When two or more hormones work together to produce a particular result, their
effects are said to be synergistic. A hormone is said to have a
permissive effect on the action of a second hormone when it enhances the
responsiveness of a target organ to the second hormone, or when it increases the
activity of the second hormone. In
some situations, the actions of one hormone antagonize the effects of another.
Lactation during pregnancy, for example, is inhibited because the high
concentration of estrogen in the blood inhibits the secretion and action of
prolactin.

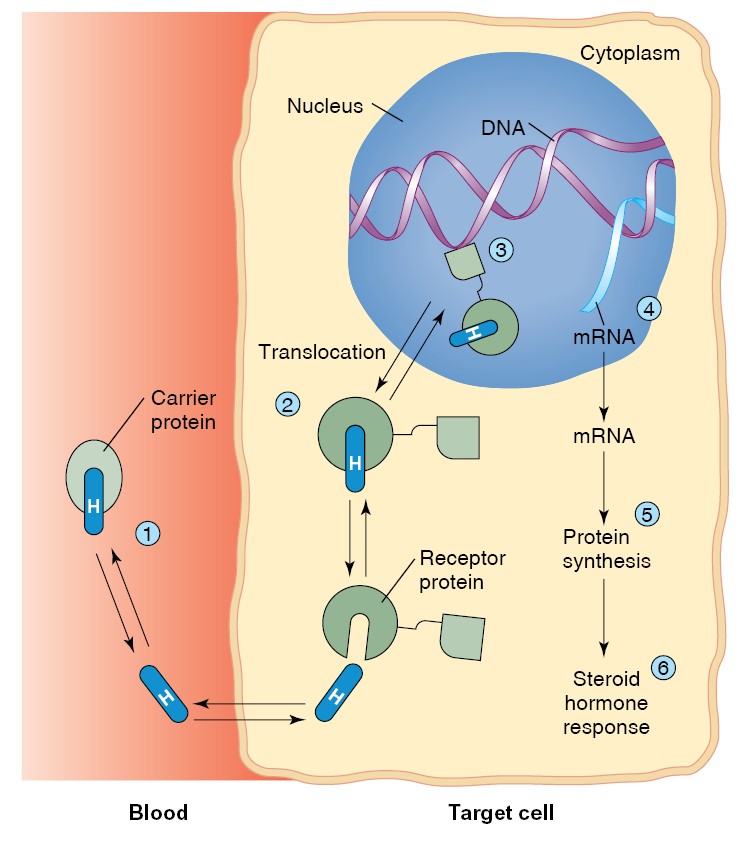
The mechanism of steroid hormone action.
(1) Steroid hormones, transported
bound to plasma carrier proteins, dissociate from their plasma carriers and pass
through the plasma membrane of their target cell. (2) The steroid hormone binds
to receptors, which may be in the cytoplasm. (3) The hormone-bound receptor
translocates to the nucleus, where it binds to DNA. (4) This stimulates genetic
transcription, resulting in new mRNA synthesis. (5) The newly formed mRNA codes
for the production of new proteins, which (6) produce the hormonal effects in
the target cell.
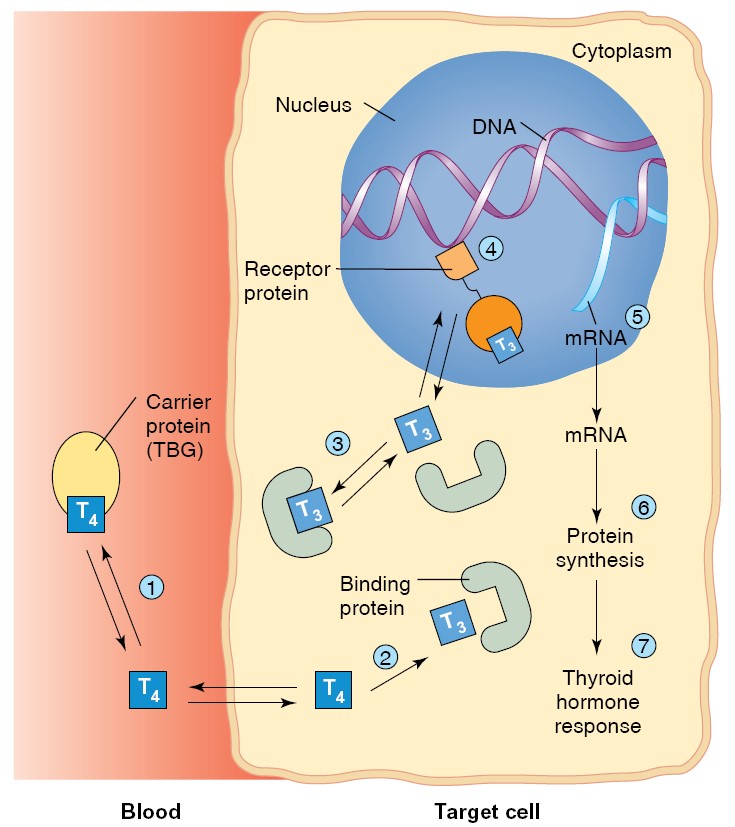
The mechanism of thyroid hormone action.
(1) Thyroxine ( T4 ), carried to
the target cell bound to its plasma carrier protein, dissociates from its
carrier and passes through the plasma membrane of its target cell. (2) In the
cytoplasm, T4 is converted into T3 (triiodothyronine), which (3) uses binding
proteins to enter the nucleus. (4) The hormone-receptor complex binds to DNA,
(5) stimulating the synthesis of new mRNA. (6) The newly formed mRNA codes for
the synthesis of new proteins, which (7) produce the hormonal effects in the
target cell.
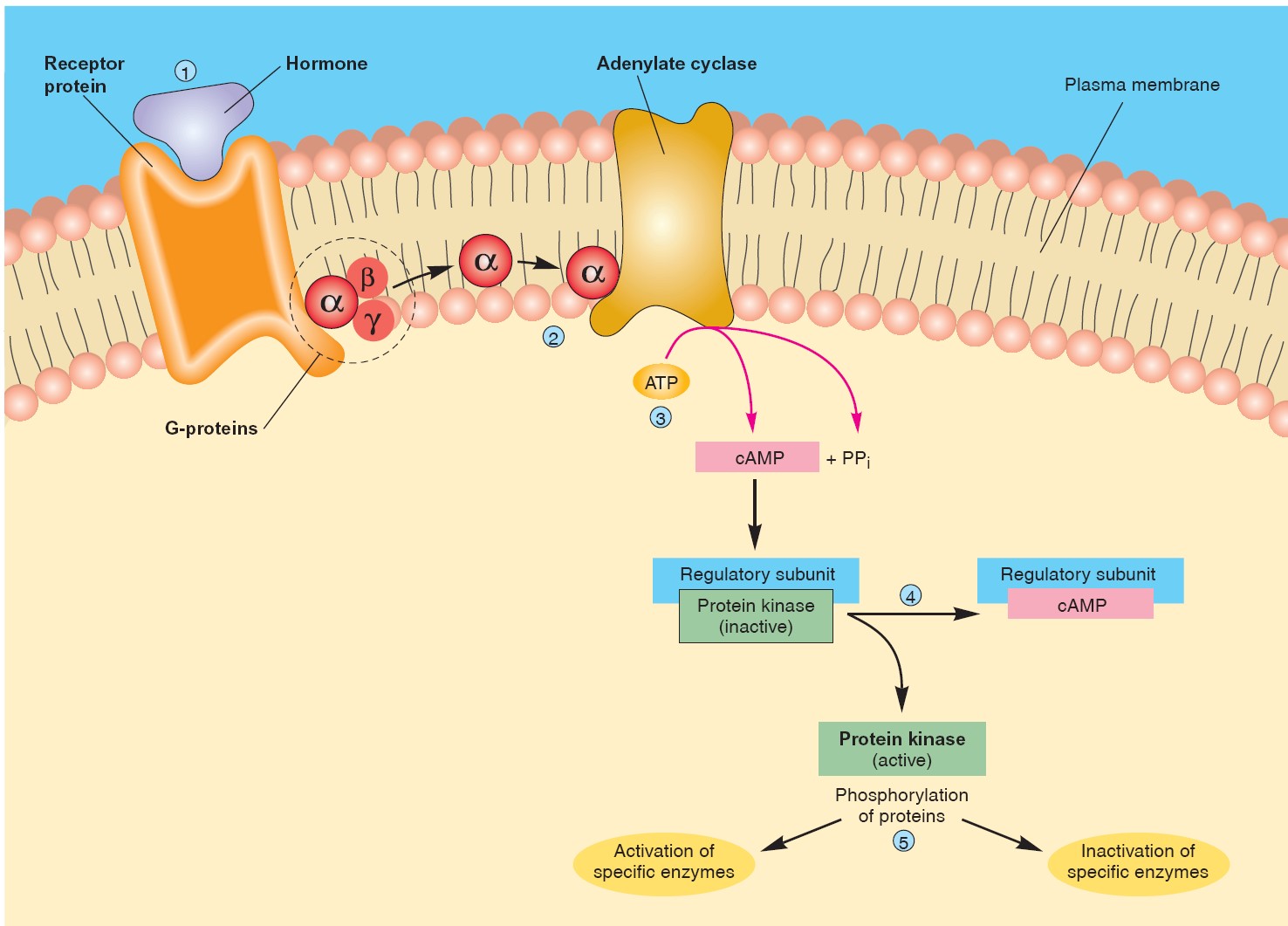
The adenylate cyclase–cyclic AMP second-messenger system.
(1) The
hormone binds to its receptor in the plasma membrane of the target cell. (2)
This causes the dissociation of G-proteins, allowing the free α (alpha) subunit
to activate adenylate cyclase. (3) This enzyme catalyzes the production of cAMP
(cyclic AMP), which (4) removes the regulatory subunit from protein kinase. (5)
Active protein kinase phosphorylates other enzyme proteins, activating or
inactivating specific enzymes and thereby producing the hormonal effects on the
target cell.
|
Table
: Endocrine Glands, Hormones, and Their Functions and Structure |
||||
|
Gland/Tissue
|
Hormones
|
Major Functions
|
Chemical Structure
|
|
|
Hypothalamus
|
Thyrotropin-releasing
|
Stimulates secretion of thyroid-stimulating |
Peptide
|
|
|
|
hormone
|
hormone and prolactin |
|
|
|
|
Corticotropin-releasing
|
Causes release of adrenocorticotropic hormone |
Peptide
|
|
|
|
hormone
|
|
|
|
|
|
Growth hormone–releasing |
Causes release of growth hormone |
Peptide
|
|
|
|
hormone
|
|
|
|
|
|
Growth hormone inhibitory |
Inhibits release of growth hormone |
Peptide
|
|
|
|
hormone (somatostatin) |
|
|
|
|
|
Gonadotropin-releasing
|
Causes release of luteinizing hormone and |
|
|
|
|
hormone
|
follicle-stimulating hormone |
|
|
|
|
Dopamine or prolactin- |
Inhibits release of prolactin |
Amine
|
|
|
|
inhibiting factor |
|
|
|
|
Anterior
|
Growth hormone |
Stimulates protein synthesis and overall growth of |
Peptide
|
|
|
pituitary
|
|
most cells and tissues |
|
|
|
|
Thyroid-stimulating hormone |
Stimulates synthesis and secretion of thyroid |
Peptide
|
|
|
|
|
hormones (thyroxine and triiodothyronine) |
|
|
|
|
Adrenocorticotropic hormone |
Stimulates synthesis and secretion of |
Peptide
|
|
|
|
|
adrenocortical hormones (cortisol, androgens, |
|
|
|
|
|
and aldosterone) |
|
|
|
|
Prolactin
|
Promotes development of the female breasts and |
Peptide
|
|
|
|
|
secretion of milk |
|
|
|
|
Follicle-stimulating hormone |
Causes growth of follicles in the ovaries and |
Peptide
|
|
|
|
|
sperm maturation in Sertoli cells of testes |
|
|
|
|
Luteinizing hormone |
Stimulates testosterone synthesis in Leydig cells of |
Peptide
|
|
|
|
|
testes; stimulates ovulation, formation of corpus |
|
|
|
|
|
luteum, and estrogen and progesterone |
|
|
|
|
|
synthesis in ovaries |
|
|
|
Posterior
|
Antidiuretic hormone (also |
Increases water reabsorption by the kidneys and |
Peptide
|
|
|
pituitary
|
called vasopressin) |
causes
|
vasoconstriction and increased blood |
|
|
|
|
pressure
|
|
|
|
|
Oxytocin
|
Stimulates milk ejection from breasts and uterine |
Peptide
|
|
|
|
|
contractions
|
|
|
|
Thyroid
|
Thyroxine (T4)and |
Increases the rates of chemical reactions in most |
Amine
|
|
|
|
triiodothyronine (T3) |
cells, thus increasing body metabolic rate |
|
|
|
|
Calcitonin
|
Promotes deposition of calcium in the bones and |
Peptide
|
|
|
|
|
decreases extracellular fluid calcium ion |
|
|
|
|
|
concentration
|
|
|
|
Adrenal cortex |
Cortisol
|
Has multiple metabolic functions for controlling |
Steroid
|
|
|
|
|
metabolism of proteins, carbohydrates, and fats; |
|
|
|
|
|
also has anti-inflammatory effects |
|
|
|
|
Aldosterone
|
Increases renal sodium reabsorption, potassium |
Steroid
|
|
|
|
|
secretion, and hydrogen ion secretion |
|
|
|
Adrenal medulla |
Norepinephrine, epinephrine |
Same effects as sympathetic stimulation |
Amine
|
|
|
|
|
|
|
|
|
Pancreas
|
Insulin (βcells) |
Promotes glucose entry in many cells, and in this |
Peptide
|
|
|
|
|
way controls carbohydrate metabolism |
|
|
|
|
Glucagon (αcells) |
Increases synthesis and release of glucose from the |
Peptide
|
|
|
|
|
liver into the body fluids |
|
|
|
Parathyroid
|
Parathyroid hormone |
Controls serum calcium ion concentration by |
Peptide
|
|
|
|
|
increasing calcium absorption by the gut and |
|
|
|
|
|
kidneys and releasing calcium from bones |
|
|
|
Testes
|
Testosterone
|
Promotes development of male reproductive |
Steroid
|
|
|
|
|
system and male secondary sexual characteristics |
|
|
|
Ovaries
|
Estrogens
|
Promotes growth and development of female |
Steroid
|
|
|
|
|
reproductive system, female breasts, and female |
|
|
|
|
|
secondary sexual characteristics |
|
|
|
|
Progesterone
|
Stimulates secretion of “uterine milk” by the |
Steroid
|
|
|
|
|
uterine endometrial glands and promotes |
|
|
|
|
|
development of secretory apparatus of breasts |
|
|
|
Placenta
|
Human chorionic |
Promotes growth of corpus luteum and secretion |
Peptide
|
|
|
gonadotropin
|
of estrogens and progesterone by corpus |
|
|
|
|
luteum
|
|
|
|
Human somatomammotropin |
Probably helps promote development of some |
Peptide
|
|
|
|
fetal tissues, as well as the mother’s breasts |
|
|
|
Estrogens
|
See actions of estrogens from ovaries |
Steroid
|
|
|
Progesterone
|
See actions of progesterone from ovaries |
Steroid
|
|
Kidney
|
Renin
|
Catalyzes conversion of angiotensinogen to |
Peptide
|
|
|
|
angiotensin I (acts as an enzyme) |
|
|
|
1,25-Dihydroxycholecalciferol
|
Increases intestinal absorption of calcium and |
Steroid
|
|
|
|
bone mineralization |
|
|
|
Erythropoietin
|
Increases erythrocyte production |
Peptide
|
|
Heart
|
Atrial natriuretic peptide |
Increases sodium excretion by kidneys, reduces |
Peptide
|
|
|
|
blood pressure |
|
|
Stomach
|
Gastrin
|
Stimulates hydrogen chloride secretion by parietal |
Peptide
|
|
|
|
cells
|
|
|
Small intestine |
Secretin
|
Stimulates pancreatic acinar cells to release |
Peptide
|
|
|
|
bicarbonate and water |
|
|
|
Cholecystokinin
|
Stimulates gallbladder contraction and release of |
Peptide
|
|
|
|
pancreatic enzymes |
|
|
Adipocytes
|
Leptin
|
Inhibits appetite, stimulates thermogenesis |
Peptide
|
|
|
|
|
|
GROWTH
HORMONE
BIOSYNTHESIS & CHEMISTRY
The long arm of human chromosome 17 contains the growth hormone-hCS cluster that
contains five genes: one, hGH-N, codes for the most abundant (“normal”)
form of growth hormone; a second, hGH-V, codes for the variant form of
growth hormone; two code for human chorionic somatomammotropin (hCS); and the
fifth is probably an hCS pseudogene.
Growth hormone that is secreted into the circulation by the pituitary
gland consists of a complex mixture of hGH-N, peptides derived from this
molecule with varying degrees of posttranslational modifications, such as
glycosylation, and a splice variant of hGH-N that lacks amino acids 32–46. The
physiologic significance of this complex array of hormones has yet to be fully
understood, particularly since their structural similarities make it difficult
to assay for each species separately. Nevertheless, there is emerging evidence
that, while the various peptides share a broad range of functions, they may
occasionally exert actions in opposition to one another. hGH-V and hCS, on the
other hand, are primarily products of the placenta, and as a consequence are
only found in appreciable quantities in the circulation during pregnancy.
SPECIES
SPECIFICITY
The structure of growth hormone varies considerably from one species to another.
Porcine and simian growth hormones have only a transient effect in the guinea
pig. In monkeys and humans, bovine and porcine growth hormones do not even have
a transient effect on growth, although monkey and human growth hormones are
fully active in both monkeys and humans. These facts are relevant to public
health discussions surrounding the presence of bovine growth hormones (used to
increase milk production) in dairy products, as well as the popularity of growth
hormone supplements, marketed via the Internet, with body builders.
Controversially, recombinant human growth hormone has also been given to
children who are short in stature, but otherwise healthy (ie, without growth
hormone deficiency), with apparently limited results.
PLASMA
LEVELS, BINDING, AND METABOLISM
A portion of circulating growth hormone is bound to a plasma protein that is a
large fragment of the extracellular domain of the growth hormone receptor. It
appears to be produced by cleavage of receptors in humans, and its concentration
is an index of the number of growth hormone receptors in the tissues.
Approximately 50% of the circulating pool of growth hormone activity is in the
bound form, providing a reservoir of the hormone to compensate for the wide
fluctuations that occur in secretion.
The basal plasma growth hormone level measured by radioimmunoassay in
adult humans is normally less than 3 ng/mL. This represents both the
protein-bound and free forms. Growth hormone is metabolized rapidly, at least in
part in the liver. The half-life of circulating growth hormone in humans is 6–20
min, and the daily growth hormone output has been calculated to be 0.2–1.0 mg/d
in adults.
GROWTH
HORMONE RECEPTORS
The growth hormone receptor is a 620-amino-acid protein with a large
extracellular portion, a transmembrane domain, and a large cytoplasmic portion.
It is a member of the cytokine receptor superfamily. Growth hormone has two
domains that can bind to its receptor, and when it binds to one receptor, the
second binding site attracts another, producing a homodimer.
Dimerization is essential for receptor
activation. Growth hormone has
widespread effects in the body, so even though it is not yet possible precisely
to correlate intracellular and whole body effects, it is not surprising that,
like insulin, growth hormone activates many different intracellular signaling
cascades. Of particular note is its activation of the JAK2–STAT pathway. JAK2 is
a member of the Janus family of cytoplasmic tyrosine kinases. STATs (for signal
transducers and activators of transcription) are a family of cytoplasmic
transcription factors that, upon phosphorylation by JAK kinases, migrate to the
nucleus where they activate various genes. JAK–STAT pathways are known also to
mediate the effects of prolactin and various other growth factors.
EFFECTS
ON GROWTH
In young animals in which the epiphyses have not yet fused to the long bones,
growth is inhibited by hypophysectomy and stimulated by growth hormone.
Chondrogenesis is accelerated, and as the cartilaginous epiphysial plates widen,
they lay down more bone matrix at the ends of long bones. In this way, stature
is increased. Prolonged treatment of animals with growth hormone leads to
gigantism. When the epiphyses are
closed, linear growth is no longer possible. In this case, an overabundance of
growth hormone produces the pattern of bone and soft tissue deformities known in
humans as acromegaly. The sizes of most of the viscera are increased. The
protein content of the body is increased, and the fat content is decreased.
EFFECTS
ON PROTEIN & ELECTROLYTE HOMEOSTASIS
Growth hormone is a protein anabolic hormone and produces a positive nitrogen
and phosphorus balance, a rise in plasma phosphorus, and a fall in blood urea
nitrogen and amino acid levels. In adults with growth hormone deficiency,
recombinant human growth hormone produces an increase in lean body mass and a
decrease in body fat, along with an increase in metabolic rate and a fall in
plasma cholesterol. Gastrointestinal absorption of Ca2+
is increased. Na+
and K+
excretion is reduced by an action independent of the adrenal glands,
probably because these electrolytes are diverted from the kidneys to the
growing tissues. On the other hand, excretion of the amino acid 4-hydroxyproline
is increased during this growth, reflective of the ability of growth hormone to
stimulate the synthesis of soluble collagen.
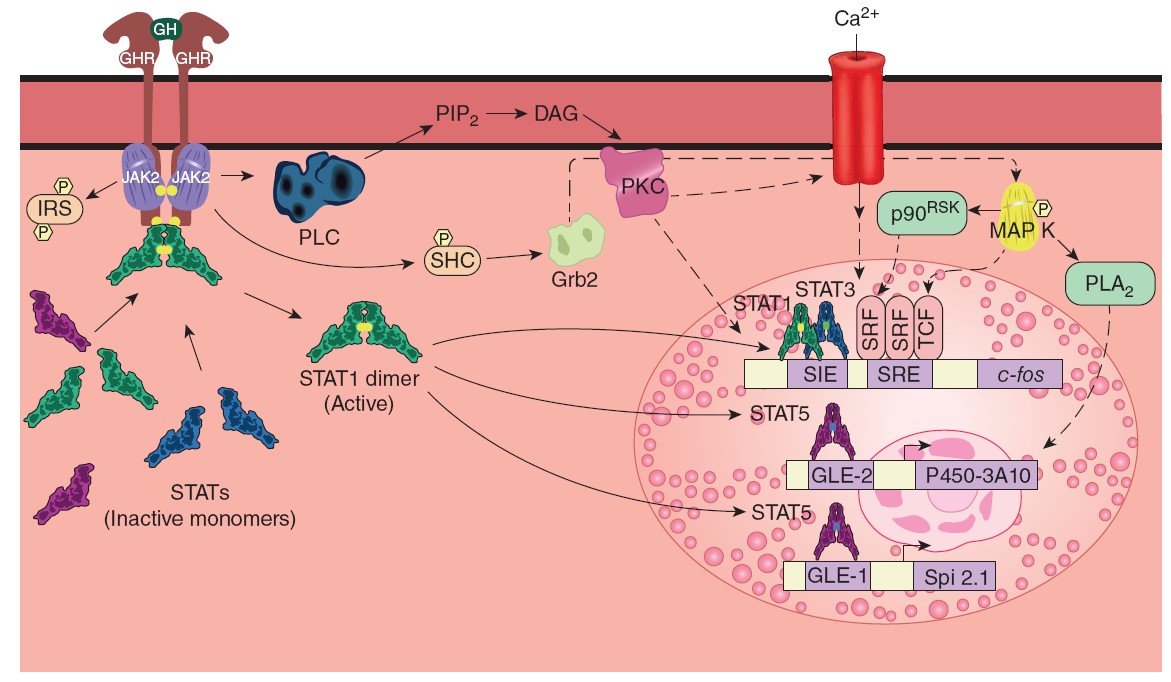
Some of the principal signaling pathways activated by the dimerized growth
hormone receptor (GHR).
Solid arrows indicate established pathways; dashed arrows
indicate probable pathways. The details of the PLC pathway and the pathway from
Grb2 to MAP K are discussed in Chapter 2. The small uppercase letter Ps in
yellow hexagons represent phosphorylation of the factor indicated. GLE-1 and
GLE-2, interferon γ-activated response elements; IRS, insulin receptor
substrate; p90RSK, an S6 kinase; PLA2,
phospholipase A2;
SIE, Sis-induced element; SRE, serum response element; SRF, serum response
factor; TCF, ternary complex factor.
EFFECTS
ON CARBOHYDRATE AND FAT METABOLISM
At least some forms of growth hormone are diabetogenic because they increase
hepatic glucose output and exert an anti-insulin effect in muscle. Growth
hormone is also ketogenic and increases circulating free fatty acid (FFA)
levels. The increase in plasma FFA, which takes several hours to develop,
provides a ready source of energy for the tissues during hypoglycemia, fasting,
and stressful stimuli. Growth hormone does not stimulate β cells of the pancreas
directly, but it increases the ability of the pancreas to respond to
insulinogenic stimuli such as arginine and glucose. This is an additional way
growth hormone promotes growth, since insulin has a protein anabolic effect.
DIRECT
AND INDIRECT ACTIONS OF GROWTH HORMONE
The understanding of the mechanism of action of growth hormone has evolved. It
was originally thought to produce growth by a direct action on tissues, and then
later was believed to act solely through its ability to induce somatomedins.
However, if growth hormone is injected into one proximal tibial epiphysis, a
unilateral increase in cartilage width is produced, and cartilage, like other
tissues, makes IGF-I. A current hypothesis to explain these results holds that
growth hormone acts on cartilage to convert stem cells into cells that respond
to IGF-I. Locally produced as well as circulating IGF-I then makes the cartilage
grow. However, the independent role of circulating IGF-I remains important,
since infusion of IGF-I in hypophysectomized rats restores bone and body growth.
Overall, it seems that growth hormone and somatomedins can act both in
cooperation and independently to stimulate pathways that lead to growth. The
situation is almost certainly complicated further by the existence of multiple
forms of growth hormone in the circulation that can, in some situations, have
opposing actions. However, growth hormone probably combines with circulating
and locally produced IGF-I in various proportions to produce at least some of
the latter effects.
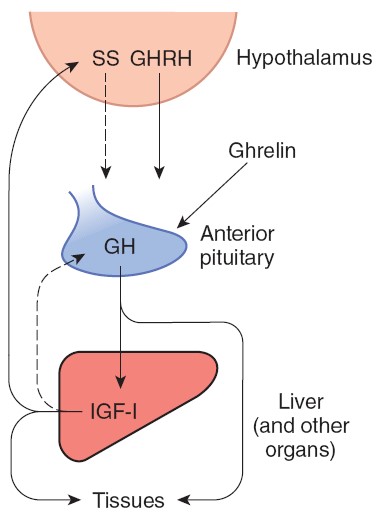
Feedback control
THYROID CELL
ANATOMIC
CONSIDERATIONS
The thyroid is a butterfly-shaped gland that straddles the trachea in the front
of the neck. It develops from an evagination of the floor of the pharynx, and a
thyroglossal duct marking the path of the thyroid from the tongue to the
neck sometimes persists in the adult. The two lobes of the human thyroid are
connected by a bridge of tissue, the thyroid isthmus, and there is
sometimes a pyramidal lobe arising from the isthmus in front of the
larynx. The gland is well vascularized, and the thyroid has one of the highest
rates of blood flow per gram of tissue of any organ in the body.
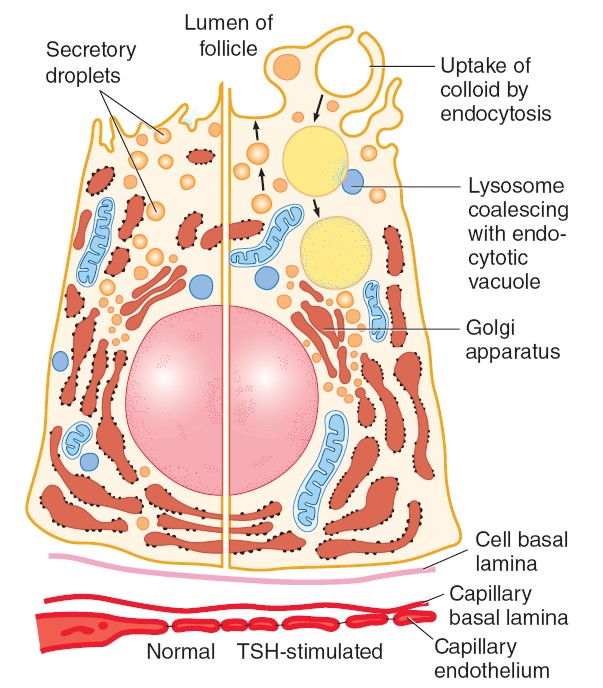
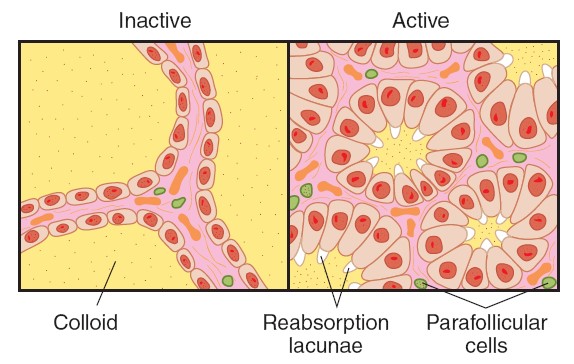
The portion of the thyroid concerned with the production of thyroid hormone
consists of multiple acini (follicles). Each spherical follicle is
surrounded by a single layer of polarized epithelial cells and filled with
pink-staining proteinaceous material called colloid. Colloid consists
predominantly of the glycoprotein, thyroglobulin. When the gland is inactive,
the colloid is abundant, the follicles are large, and the cells lining them are
flat. When the gland is active, the follicles are small, the cells are cuboid or
columnar, and areas where the colloid is being actively reabsorbed into the
thyrocytes are visible as “reabsorption lacunae.
Microvilli project into the colloid from the apexes of the thyroid cells
and canaliculi extend into them. The endoplasmic reticulum is prominent, a
feature common to most glandular cells, and secretory granules containing
thyroglobulin are seen. The individual thyroid cells rest on a basal lamina that
separates them from the adjacent capillaries. The capillaries are fenestrated,
like those of other endocrine glands.
FORMATION AND SECRETION OF THYROID HORMONES
THYROID HORMONE SYNTHESIS AND SECRETION
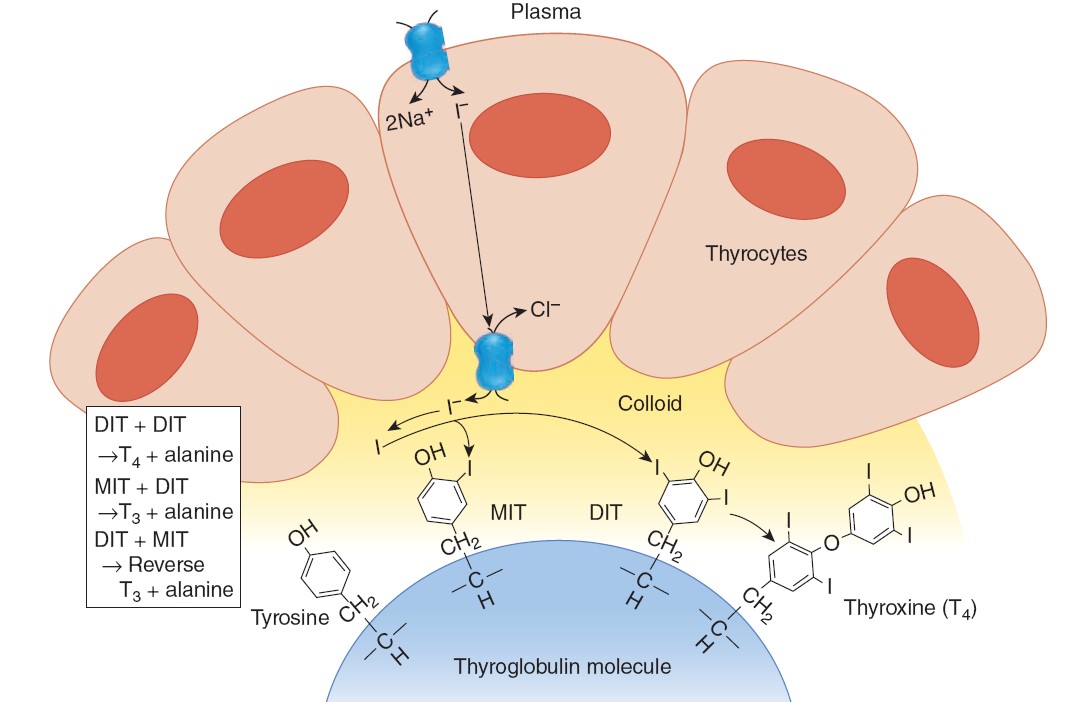
At the interface between the thyrocyte and the colloid, iodide undergoes a
process referred to as organification. First, it is oxidized to iodine, and then
incorporated into the carbon 3 position of tyrosine residues that are part of
the thyroglobulin molecule in the colloid. Thyroglobulin is a
glycoprotein made up of two subunits and has a molecular weight of 660 kDa. It
contains 10% carbohydrate by weight. It also contains 123 tyrosine residues, but
only 4–8 of these are normally incorporated into thyroid hormones. Thyroglobulin
is synthesized in the thyroid cells and secreted into the colloid by exocytosis
of granules. The oxidation and reaction of iodide with the secreted
thyroglobulin is mediated by thyroid peroxidase, a membrane-bound enzyme
found in the thyrocyte apical membrane. The thyroid hormones so produced remain
part of the thyroglobulin molecule until needed. As such, colloid represents a
reservoir of thyroid hormones, and humans can ingest a diet completely devoid of
iodide for up to 2 months before a decline in circulating thyroid hormone levels
is seen. When there is a need for thyroid hormone secretion, colloid is
internalized by the thyrocytes by endocytosis, and directed toward lysosomal
degradation. Thus, the peptide bonds of thyroglobulin are hydrolyzed, and free
T4 and T3 are discharged into cytosol and thence to the capillaries (see below).
Thyrocytes thus have four functions: They collect and transport iodine, they
synthesize thyroglobulin and secrete it into the colloid, they fix iodine to the
thyroglobulin to generate thyroid hormones, and they remove the thyroid
hormones from thyroglobulin and secrete them into the circulation.
Thyroid hormone synthesis is a multistep process. Thyroid peroxidase generates
reactive iodine species that can attack thyroglobulin. The first product is
monoiodotyrosine (MIT). MIT is next iodinated on the carbon 5 position to form
diiodotyrosine (DIT). Two DIT molecules then undergo an oxidative condensation
to form T4 with the elimination of the alanine side chain from the molecule that
forms the outer ring. There are two theories of how this coupling reaction
occurs. One holds that the coupling occurs with both DIT molecules attached
to thyroglobulin (intramolecular coupling). The other holds that the DIT that
forms the outer ring is first detached from thyroglobulin (intermolecular
coupling). In either case, thyroid peroxidase is involved in coupling as well as
iodination. T3 is formed by condensation of MIT with DIT. A small amount of RT3
is also formed, probably by condensation of DIT with MIT. In the normal human
thyroid, the average distribution of iodinated compounds is 3% MIT, 33% DIT, 35%
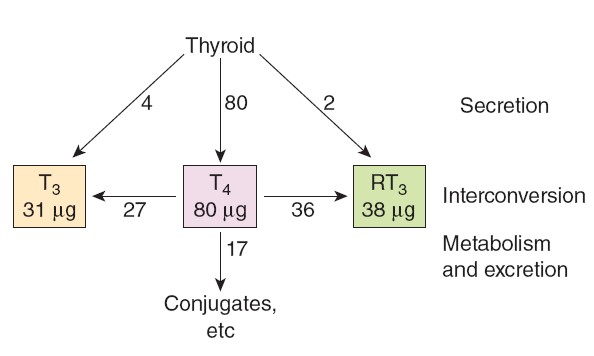
T4, and 7% T3. Only traces of RT3 and other components are present.
The human thyroid secretes about 80 μg (103 nmol) of T4, 4 μg (7 nmol) of T3,
and 2 μg (3.5 nmol) of RT3 per day. MIT and DIT are not secreted. These
iodinated tyrosines are deiodinated by a microsomal iodotyrosine deiodinase.
This represents a mechanism to recover iodine and bound tyrosines and
recycle them for additional rounds of hormone synthesis. The iodine liberated by
deiodination of MIT and DIT is reutilized in the gland and normally provides
about twice as much iodide for hormone synthesis as NIS does. In patients with
congenital absence of the iodotyrosine deiodinase, MIT and DIT appear in the
urine and there are symptoms of iodine deficiency.
Iodinated thyronines are resistant to the activity of
iodotyrosine deiodinase, thus allowing T4 and T3 to pass into the circulation.
MECHANISM OF ACTION
Thyroid hormones enter cells and T3 binds to TR in the nuclei. T4 can also bind,
but not as avidly. The hormone–receptor complex then binds to DNA via zinc
fingers and increases (or in some cases, decreases) the expression of a variety
of different genes that code for proteins that regulate cell function. Thus,
the nuclear receptors for thyroid hormones are members of the superfamily of
hormone-sensitive nuclear transcription factors.There are two human TR genes: an
α receptor gene on chromosome 17 and a β receptor gene on chromosome 3.
By alternative splicing, each forms at
least two different mRNAs and therefore two different receptor proteins. TRβ2 is
found only in the brain, but TRα1, TRα2, and TRβ1 are widely distributed. TRα2
differs from the other three in that it does not bind T3 and its function is not
yet fully established. TRs bind to DNA as monomers, homodimers, and heterodimers
with other nuclear receptors, particularly the retinoid X receptor (RXR).
The TR/RXR heterodimer does not bind to 9-cis retinoic acid, the usual
ligand for RXR, but TR binding to DNA is greatly enhanced in response to thyroid
hormones when the receptor is in the form of this heterodimer. There are also
coactivator and corepressor proteins that affect the actions of TRs. Presumably,
this complexity underlies the ability of thyroid hormones to produce many
different effects in the body. In
most of its actions, T3 acts more rapidly and is three to five times more potent
than T4. This is because T3 is less tightly bound to plasma proteins than is T4,
but binds more avidly to thyroid hormone receptors. As previously noted, RT3 is
inert.
CALORIGENIC ACTION
T4 and T3 increase the O2 consumption of almost all metabolically active
tissues. The exceptions are the adult brain, testes, uterus, lymph nodes,
spleen, and anterior pituitary. T4 actually depresses the O2 consumption of the
anterior pituitary, presumably because it inhibits TSH secretion. The increase
in metabolic rate produced by a single dose of T4 becomes measurable after a
latent period of several hours and lasts 6 days or more.
Some of the calorigenic effect of thyroid hormones is due to metabolism of the
fatty acids they mobilize. In addition, thyroid hormones increase the activity
of the membrane-bound Na, K ATPase in many tissues.
EFFECTS ON THE CARDIOVASCULAR SYSTEM
Large doses of thyroid hormones cause enough extra heat production to lead to a
slight rise in body temperatures, which in turn activates heat-dissipating
mechanisms. Peripheral resistance decreases because of cutaneous vasodilation,
and this increases levels of renal Na+ and water absorption, expanding blood
volume. Cardiac output is increased by the direct action of thyroid hormones, as
well as that of catecholamines, on the heart, so that pulse pressure and
cardiac rate are increased and circulation time is shortened.
T3 is not formed from T4 in cardiac myocytes to any degree, but circulatory T3
enters the myocytes, combines with its receptors, and enters the nucleus, where
it promotes the expression of some genes and inhibits the expression of others.
Those that are enhanced include the genes for α-myosin heavy chain, sarcoplasmic
reticulum Ca2+ ATPase, β-adrenergic receptors, G-proteins, Na, K ATPase, and
certain K+ channels. Those that are inhibited include the genes for β-myosin
heavy chain, phospholamban, two types of adenylyl cyclase, T3 nuclear receptors,
and NCX, the Na+–Ca2+ exchanger. The net result is increased heart rate and
force of contraction.
The two myosin heavy chain (MHC) isoforms, α-MHC and β-MHC, produced by the
heart are encoded by two highly homologous genes located on the short arm of
chromosome 17. Each myosin molecule consists of two heavy chains and two pairs
of light chains. The myosin containing β-MHC has less ATPase activity than the
myosin containing α-MHC. α-MHC predominates in the atria in adults, and its
level is increased by treatment with thyroid hormone. This increases the speed
of cardiac contraction. Conversely, expression of the α-MHC gene is depressed
and that of the β-MHC gene is enhanced in hypothyroidism.
EFFECTS ON THE NERVOUS SYSTEM
In hypothyroidism, mentation is slow and the cerebrospinal fluid (CSF) protein
level is elevated. Thyroid hormones reverse these changes, and large doses cause
rapid mentation, irritability, and restlessness. Overall, cerebral blood flow
and glucose and O2 consumption by the brain are normal in adult hypothyroidism
and hyperthyroidism. However, thyroid hormones enter the brain in adults and
are found in gray matter in numerous different locations. In addition,
astrocytes in the brain convert T4 to T3, and there is a sharp increase in brain
D2 activity after thyroidectomy that is reversed within 4 h by a single
intravenous dose of T3. Some of the effects of thyroid hormones on the brain are
probably secondary to increased responsiveness to catecholamines, with
consequent increased activation of the reticular activating system. In addition,
thyroid hormones have marked effects on brain development. The parts of the
central nervous system (CNS) most affected are the cerebral cortex and the basal
ganglia. In addition, the cochlea is also affected. Consequently, thyroid
hormone deficiency during development causes mental retardation, motor
rigidity, and deaf–mutism. Deficiencies in thyroid hormone synthesis secondary
to a failure of thyrocytes to transport iodide presumably also contribute to
deafness in Pendred syndrome.
Thyroid hormones also exert effects on reflexes. The reaction time of stretch
reflexes is shortened in hyperthyroidism and prolonged in hypothyroidism.
Measurement of the reaction time of the ankle jerk (Achilles reflex) has
attracted attention as a clinical test for evaluating thyroid function, but this
reaction time is also affected by other diseases and thus is not a specific
assessment of thyroid activity.
RELATION TO CATECHOLAMINES
The actions of thyroid hormones and the catecholamines norepinephrine and
epinephrine are intimately interrelated. Epinephrine increases the metabolic
rate, stimulates the nervous system, and produces cardiovascular effects similar
to those of thyroid hormones, although the duration of these actions is brief.
Norepinephrine has generally similar actions. The toxicity of the catecholamines
is markedly increased in rats treated with T4. Although plasma catecholamine
levels are normal in hyperthyroidism, the cardiovascular effects,
tremulousness, and sweating that are seen in the setting of excess thyroid
hormones can be reduced or abolished by sympathectomy. They can also be reduced
by drugs such as propranolol that block β-adrenergic receptors. Indeed,
propranolol and other β-blockers are used extensively in the treatment of
thyrotoxicosis and in the treatment of the severe exacerbations of
hyperthyroidism called thyroid storms. However, even though β-blockers
are weak inhibitors of extrathyroidal conversion of T4 to T3, and consequently
may produce a small fall in plasma T3, they have little effect on the other
actions of thyroid hormones. Presumably, the functional synergism observed
between catecholamines and thyroid hormones, particularly in pathologic
settings, arises from their overlapping biologic functions as well as the
ability of thyroid hormones to increase expression of catecholamine receptors
and the signaling effectors to which they are linked.
EFFECTS ON SKELETAL MUSCLE
Muscle weakness occurs in most patients with hyperthyroidism (thyrotoxic
myopathy), and when the hyperthyroidism is severe and prolonged, the
myopathy may be severe. The muscle weakness may be due in part to increased
protein catabolism. Thyroid hormones affect the expression of the MHC genes in
skeletal as well as cardiac muscle. However, the effects produced are complex
and their relation to the myopathy is not established. Hypothyroidism is also
associated with muscle weakness, cramps, and stiffness.
EFFECTS ON CARBOHYDRATE METABOLISM
Thyroid hormones increase the rate of absorption of carbohydrates from the
gastrointestinal tract, an action that is probably independent of their
calorigenic action. In hyperthyroidism, therefore, the plasma glucose level
rises rapidly after a carbohydrate meal, sometimes exceeding the renal
threshold. However, it falls again at a rapid rate.
EFFECTS ON CHOLESTEROL METABOLISM
Thyroid hormones lower circulating cholesterol levels. The plasma cholesterol
level drops before the metabolic rate rises, which indicates that this action is
independent of the stimulation of O2 consumption. The decrease in plasma
cholesterol concentration is due to increased formation of low-density
lipoprotein (LDL) receptors in the liver, resulting in increased hepatic removal
of cholesterol from the circulation. Despite considerable effort, however, it
has not been possible to produce a clinically useful thyroid hormone analog
that lowers plasma cholesterol without increasing metabolism.
EFFECTS
ON GROWTH
Thyroid hormones are essential for normal growth and skeletal maturation. In
hypothyroid children, bone growth is slowed and epiphysial closure delayed. In
the absence of thyroid hormones, growth hormone secretion is also depressed.
This further impairs growth and development, since thyroid hormones normally
potentiate the effect of growth hormone on tissues.
ADRENAL GLANDS
There are two endocrine organs in the adrenal gland, one surrounding the other.
The main secretions of the inner adrenal medulla
are the catecholamines epinephrine, norepinephrine, and dopamine;
the outer adrenal cortex secretes steroid hormones.
The adrenal medulla is in effect a sympathetic ganglion in which the
postganglionic neurons have lost their axons and become secretory cells. The
cells secrete when stimulated by the preganglionic nerve fibers that reach the
gland via the splanchnic nerves. Adrenal medullary hormones work mostly to
prepare the body for emergencies, the so-called “fight-or-flight” responses.
The adrenal cortex secretes glucocorticoids, steroids with
widespread effects on the metabolism of carbohydrate and protein; and a
mineralocorticoid essential to the maintenance of Na+ balance and
extracellular fluid (ECF) volume. It is also a secondary site of androgen
synthesis, secreting sex hormones such as testosterone, which can exert effects
on reproductive function. Mineralocorticoids and the glucocorticoids are
necessary for survival. Adrenocortical secretion is controlled primarily by
adrenocorticotropic hormone (ACTH) from the anterior pituitary, but
mineralocorticoid secretion is also subject to independent control by
circulating factors, of which the most important is angiotensin II, a
peptide formed in the bloodstream by the action of renin.
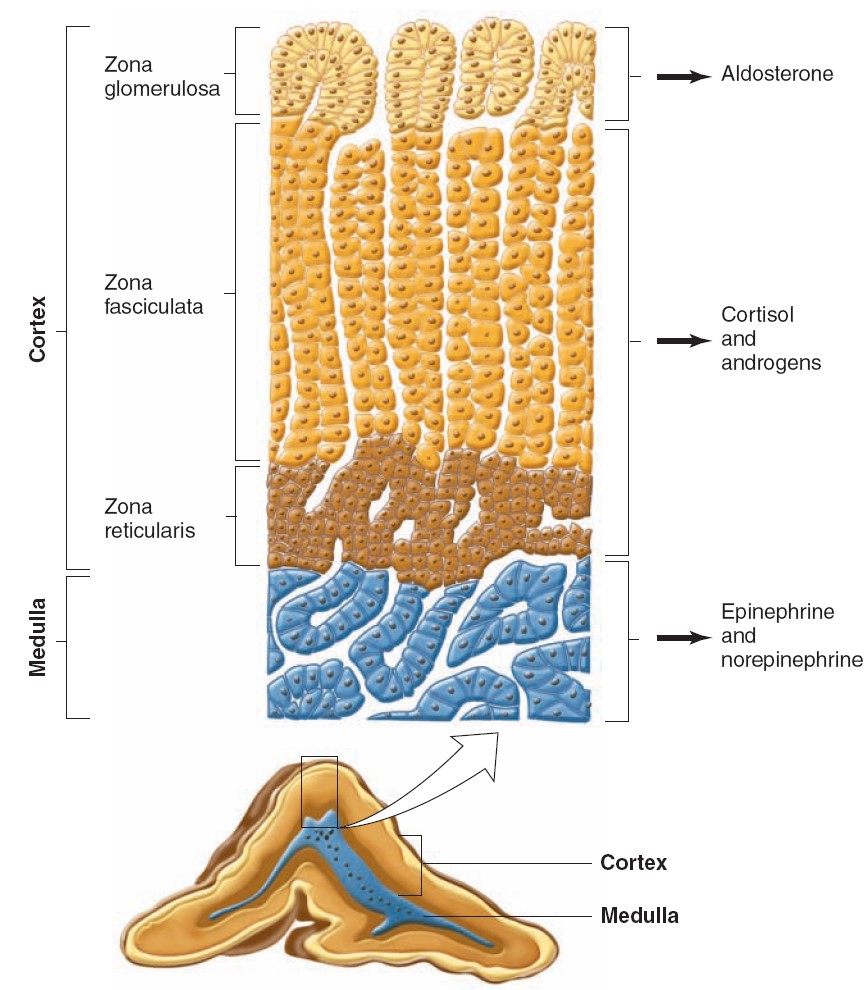
ADRENAL CORTEX: STRUCTURE & BIOSYNTHESIS OF ADRENOCORTICAL HORMONES
The hormones of the adrenal cortex are derivatives of cholesterol. Like
cholesterol, bile acids, vitamin D, and ovarian and testicular steroids, they
contain the cyclopentanoperhydrophenanthrene nucleus. Gonadal and
adrenocortical steroids are of three types: C21
steroids, which have a two-carbon side chain at position 17; C19
steroids, which
have a two-carbon side chain at position 17; C 19 steroids,
which have a keto or hydroxyl group at position 17; and C 18 steroids, which, in
addition to a 17-keto or hydroxyl group, have no angular methyl group attached
to position 10. The adrenal cortex secretes primarily C 21 and C 19 steroids.
Most of the C 19 steroids have a keto group at position 17 and are therefore
called 17-ketosteroids. The C 21 steroids that have a hydroxyl group at
the 17 position in addition to the side chain are oft en called
17-hydroxycorticoids or 17-hydroxycorticosteroids. The C 19 steroids have
androgenic activity. The C 21 steroids are classified, using Selye’s
terminology, as mineralocorticoids or glucocorticoids. All secreted C 21
steroids have both mineralocorticoid and glucocorticoid activity;
mineralocorticoids are those in which effects on Na + and K + excretion
predominate and glucocorticoids are those in which effects on glucose and
protein metabolism predominate.
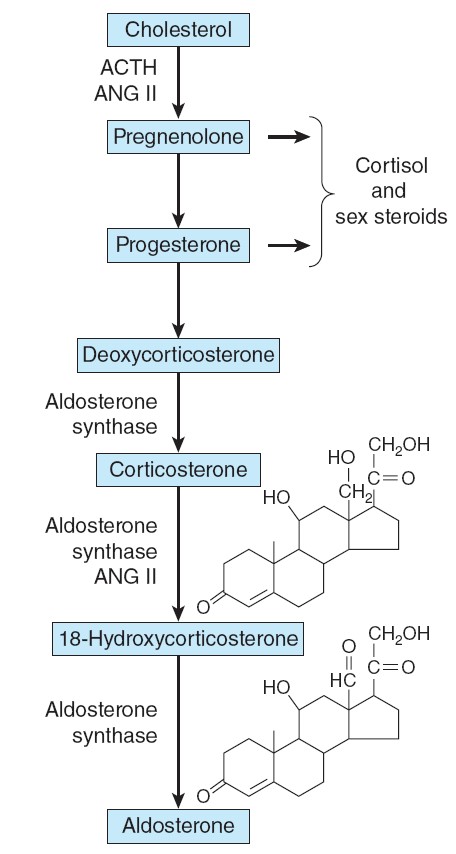
Hormone synthesis in the zona glomerulosa.
The zona glomerulosa lacks 17α-hydroxylase activity, and only the
zona glomerulosa can convert corticosterone to aldosterone because it is the
only zone that normally contains aldosterone synthase. ANG II, angiotensin II.
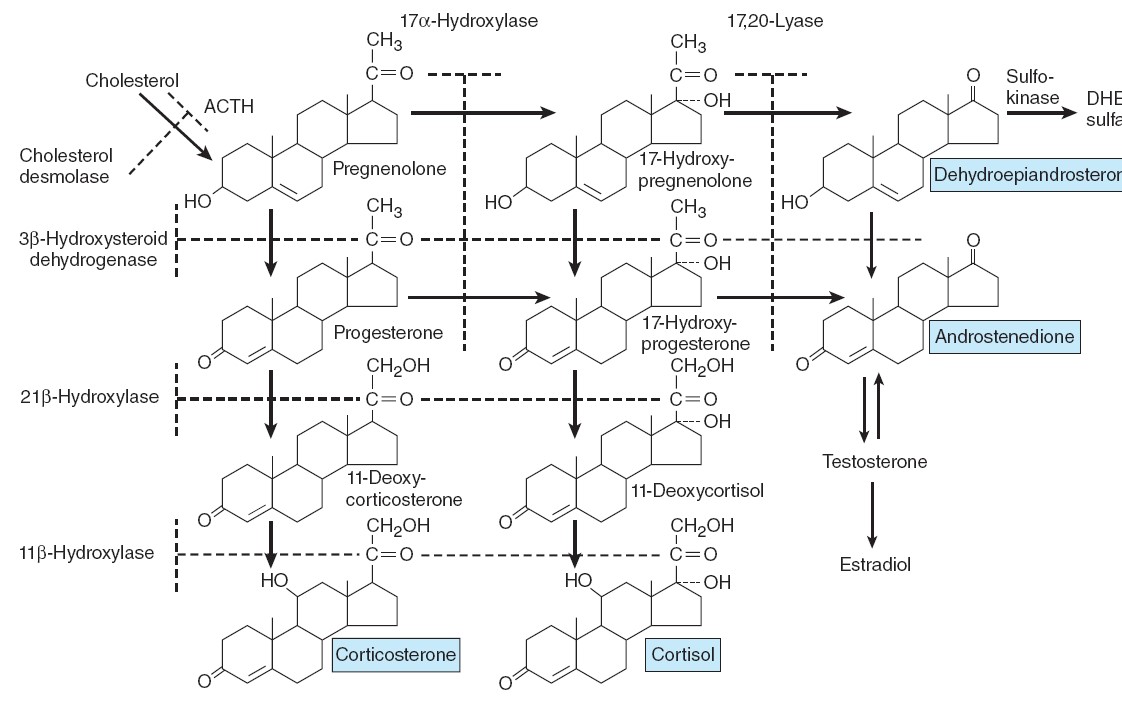
Outline of hormone biosynthesis in the zona fasciculata and zona reticularis of
the adrenal cortex.
The major secretory products are underlined. The enzymes for the
reactions are shown on the left and at the top of the chart. When a particular
enzyme is deficient, hormone production is blocked at the points indicated by
the shaded bars.
EFFECTS OF MINERALOCORTICOIDS ACTIONS
Aldosterone and other steroids with mineralocorticoid activity increase the
reabsorption of Na+ from the urine, sweat, saliva, and the contents of the
colon. Thus, mineralocorticoids cause retention of Na+ in the ECF. This expands
ECF volume. In the kidneys, they act primarily on the principal cells (P
cells) of the collecting ducts. Under the influence of aldosterone,
increased amounts of Na+ are in effect exchanged for K+ and H+ in the renal
tubules, producing a K+ diuresis and an increase in urine acidity.
MECHANISM OF ACTION
Like many other steroids, aldosterone binds to a cytoplasmic receptor, and the
receptor–hormone complex moves to the nucleus where it alters the transcription
of mRNAs. This in turn increases the production of proteins that alter cell
function. The aldosterone-stimulated proteins have two effects—a rapid effect,
to increase the activity of epithelial sodium channels (ENaCs) by increasing
the insertion of these channels into the cell membrane from a cytoplasmic pool;
and a slower effect to increase the synthesis of ENaCs. Among the genes
activated by aldosterone is the gene for serum-and glucocorticoid regulated
kinase (sgk), a serine-threonine protein kinase. The gene for sgk is an
early response gene, and sgk increases ENaC activity. Aldosterone also increases
the mRNAs for the three
subunits that make up ENaCs. The fact that sgk is activated by
glucocorticoids as well as aldosterone is not a problem because glucocorticoids
are inactivated at mineralocorticoid receptor sites. However, aldosterone
activates the genes for other proteins in addition to sgk and ENaCs and
inhibits others. Therefore, the exact mechanism by which aldosterone-induced
proteins increase Na+
reabsorption is
still unsettled. Evidence is
accumulating that aldosterone also binds to the cell membrane and by a rapid,
nongenomic action increases the activity of membrane Na+–K+
exchangers. This produces an increase in intracellular Na+,
and the second messenger involved is probably IP3.
In any case, the principal effect of aldosterone on Na+
transport takes 10–30 min to develop and peaks even later, indicating that it
depends on the synthesis of new proteins by a genomic mechanism.
OTHER STEROIDS THAT AFFECT Na+ EXCRETION
Aldosterone is the principal mineralocorticoid secreted by the adrenals,
although corticosterone is secreted in sufficient amounts to exert a minor
mineralocorticoid effect. Deoxycorticosterone, which is secreted in appreciable
amounts only in abnormal situations, has about 3% of the activity of
aldosterone. Large amounts of progesterone and some other steroids cause
natriuresis, but there is little evidence that they play any normal role in the
control of Na+ excretion.
EFFECT OF ADRENALECTOMY
In adrenal insufficiency, Na+ is lost in the urine; K+ is retained, and the
plasma K+ rises. When adrenal insufficiency develops rapidly, the amount of Na+
lost from the ECF exceeds the amount excreted in the urine, indicating that Na+
also must be entering cells. When the posterior pituitary is intact, salt loss
exceeds water loss, and the plasma Na+ falls. However, the plasma volume is also
reduced, resulting in hypotension, circulatory insufficiency and, eventually,
fatal shock. These changes can be prevented to a degree by increasing dietary
NaCl intake. Rats survive indefinitely on extra salt alone, but in dogs and most
humans, the amount of supplementary salt needed is so large that it is almost
impossible to prevent eventual collapse and death unless mineralocorticoid
treatment is also instituted.
ROLE OF
MINERALOCORTICOIDS IN THE REGULATION OF SALT BALANCE
Variations in aldosterone secretion is only one of many factors affecting Na+
excretion. Other major factors include the glomerular filtration rate, ANP, the
presence or absence of osmotic diuresis, and changes in tubular reabsorption of
Na+
independent of aldosterone. It takes some time for aldosterone to
act. When one rises from the supine to the standing position, aldosterone
secretion increases and Na+
is retained from the urine. However, the decrease in Na+
excretion develops too rapidly to be explained solely by
increased aldosterone secretion. The primary function of the
aldosterone-secreting mechanism is the defense of intravascular volume, but it
is only one of the homeostatic mechanisms involved in this regulation.
ESTROGENS
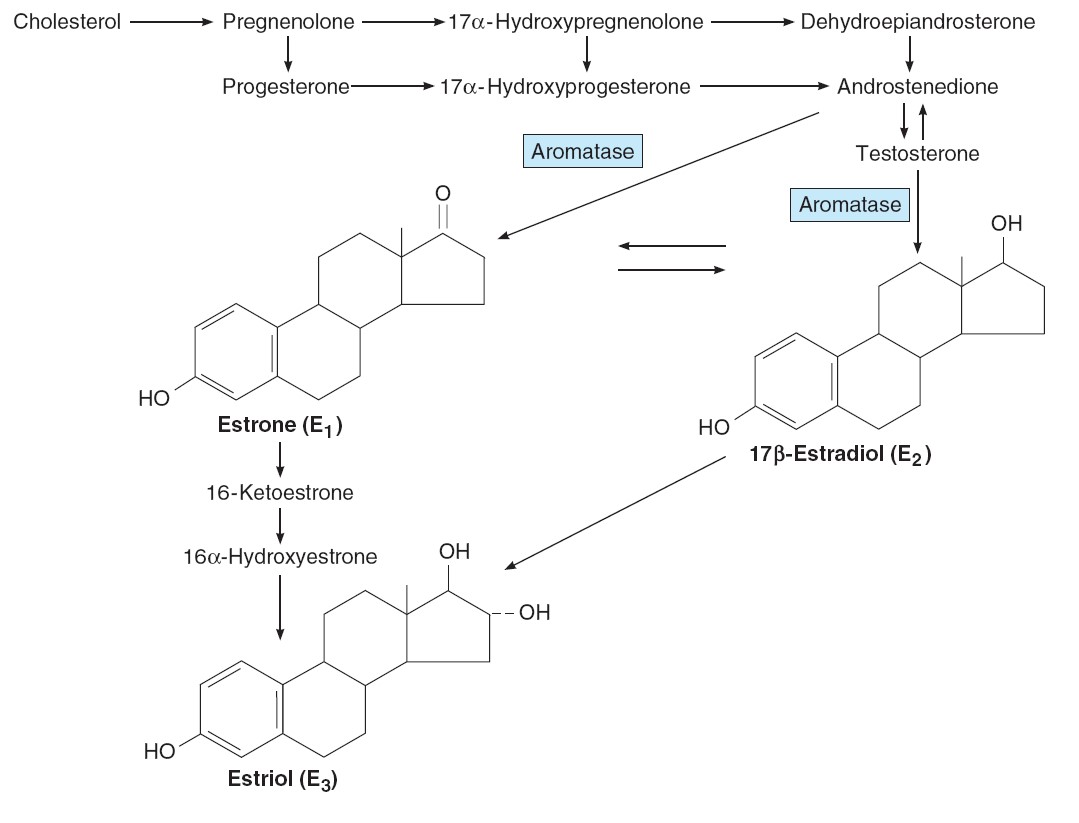
Chemistry, Biosynthesis, & Metabolism of Estrogens
The naturally occurring estrogens are 17β-estradiol, estrone, and
estriol. They are C18 steroids that do not have an angular methyl group
attached to the 10 position or a ê4-3-keto configuration in the A ring. They are
secreted primarily by the granulosa cells of the ovarian follicles, the corpus
luteum, and the placenta. Their biosynthesis depends on the enzyme aromatase
(CYP19), which converts testosterone to estradiol and androstenedione to
estrone. The latter reaction also occurs in fat, liver, muscle, and the brain.
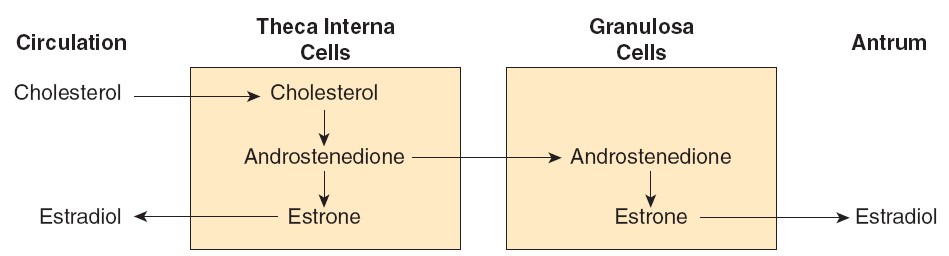
Theca interna cells have many LH receptors, and LH acts via cAMP to increase
conversion of cholesterol to androstenedione. The theca interna cells supply
androstenedione to the granulosa cells. The granulosa cells make estradiol when
provided with androgens, and it appears that the estradiol they form in primates
is secreted into the follicular fluid. Granulosa cells have many FSH receptors,
and FSH facilitates their secretion of estradiol by acting via cAMP to increase
their aromatase activity. Mature granulosa cells also acquire LH receptors, and
LH also stimulates estradiol production.
Two percent of the circulating estradiol is free, and the remainder is
bound to protein: 60% to albumin and 38% to the same gonadal steroid-binding
globulin (GBG) that binds testosterone.
In the liver, estradiol, estrone, and estriol are converted to
glucuronide and sulfate conjugates. All these compounds, along with other
metabolites, are excreted in the urine. Appreciable amounts are secreted in the
bile and reabsorbed into the bloodstream (enterohepatic circulation).
Secretion
The concentration of estradiol in the plasma during the menstrual cycle is
shown in Figure 22–14. Almost all of this estrogen comes from the ovary, and
two peaks of secretion occur: one just before ovulation and one during the
midluteal phase. The estradiol secretion rate is 36 μg/day (133 nmol/day) in the
early follicular phase, 380 μg/day just before ovulation, and 250 μg/day during
the midluteal phase. After menopause, estrogen secretion declines to low levels.
As noted previously, the estradiol production rate in men is about 50
μg/day (184 nmol/day).
Effects on the Female Genitalia
Estrogens facilitate the growth of the ovarian follicles and increase the
motility of the uterine tubes. Their role in the cyclic changes in the
endometrium, cervix, and vagina has been discussed previously. They increase
uterine blood flow and have important effects on the smooth muscle of the
uterus.
In immature and castrated females, the uterus is small and the myometrium
atrophic and inactive. Estrogens increase the amount of uterine muscle and its
content of contractile proteins. Under the influence of estrogens, the muscle
becomes more active and excitable, and action potentials in the individual
fibers become more frequent. The “estrogen-dominated” uterus is also more
sensitive to oxytocin.
Chronic treatment with estrogens causes the endometrium to hypertrophy. When
estrogen therapy is discontinued, sloughing takes place with withdrawal
bleeding. Some “breakthrough” bleeding may occur during treatment when
estrogens are given for long periods. Prolonged exposure to estrogen alone
(unopposed by progesterone) has been indicated as a risk factor in the
development of endometrial cancer.
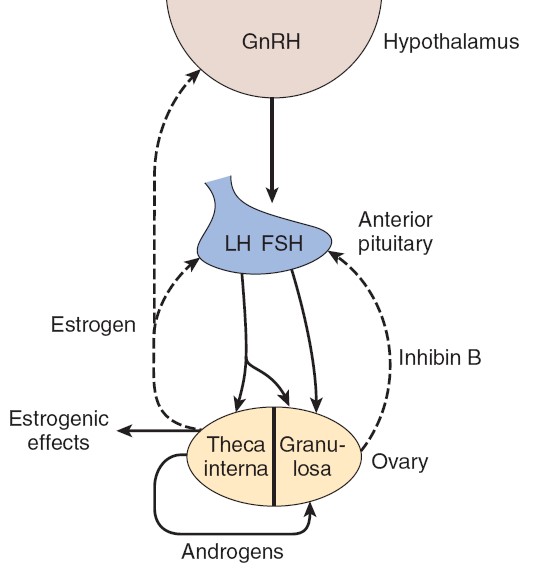
Effects on Endocrine Organs
Estrogens decrease FSH secretion. Under some circumstances, they inhibit LH
secretion (negative feedback); in other circumstances, they increase LH
secretion (positive feedback). Women are sometimes given large doses of
estrogens for 4–6 days to prevent conception after coitus during the fertile
period (postcoital or “morning-after” contraception). However, in this instance,
pregnancy is probably prevented by interference with implantation of the ovum
rather than changes in gonadotropin secretion.
Estrogens cause increased secretion of angiotensinogen and thyroid-binding
globulin. They exert an important protein anabolic effect in chickens and
cattle, possibly by stimulating the secretion of androgens from the adrenal,
and estrogen treatment has been used commercially to increase the weight of
domestic animals. They cause epiphysial closure in humans.
Effects on the Central Nervous System
The estrogens are responsible for estrous behavior in animals, and they increase
libido in humans. They apparently exert this action by a direct effect on
certain neurons in the hypothalamus. Estrogens also increase the proliferation
of dendrites on neurons and the number of synaptic knobs in rats.
Effects on the Breasts
Estrogens produce duct growth in the breasts and are largely responsible for
breast enlargement at puberty in girls; they have been called the growth
hormones of the breast. They are responsible for the pigmentation of the
areolas, although pigmentation usually becomes more intense during the first
pregnancy than it does at puberty.
Female Secondary Sex Characteristics
The body changes that develop in girls at puberty—in addition to enlargement of
breasts, uterus, and vagina—are due in part to estrogens, which are the
“feminizing hormones,” and in part simply to the absence of testicular
androgens. Women have narrow shoulders and broad hips, thighs that converge,
and arms that diverge (wide carrying angle). This body
configuration, plus the female distribution of fat in the breasts and buttocks,
is seen also in castrate males. In women, the larynx retains its prepubertal
proportions and the voice stays high-pitched. Women have less body hair and more
scalp hair, and the pubic hair generally has a characteristic flat-topped
pattern (female escutcheon). However, growth of pubic and axillary hair in both
sexes is due primarily to androgens rather than estrogens.
Other Actions
Normal women retain salt and water and gain weight just before menstruation.
Estrogens cause some degree of salt and water retention. However, aldosterone
secretion is slightly elevated in the luteal phase, and this also contributes to
the premenstrual fluid retention.
Estrogens are said to make sebaceous gland secretions more fluid and thus to
counter the effect of testosterone and inhibit formation of comedones
(“black-heads”) and acne. The liver palms, spider angiomas, and slight breast
enlargement seen in advanced liver disease are due to increased circulating
estrogens. The increase appears to be due to decreased hepatic metabolism of
androstenedione, making more of this androgen available for conversion to
estrogens. Estrogens have a
significant plasma cholesterol-lowering action, and they rapidly produce
vasodilation by increasing the local production of NO.
Mechanism of Action
There are two principal types of nuclear estrogen receptors: estrogen receptor α
(ERα) encoded by a gene on chromosome 6; and estrogen receptor β (ERβ), encoded
by a gene on chromosome 14. Both are members of the nuclear receptor
super-family. After binding estrogen, they form homodimers and bind to DNA,
altering its transcription. Some tissues contain one type or the other, but
overlap also occurs, with some tissues containing both ERα and ERβ. ERα is found
primarily in the uterus, kidneys, liver, and heart, whereas ERβ is found
primarily in the ovaries, prostate, lungs, gastrointestinal tract, hemopoietic
system, and central nervous system (CNS). ERα and ERβ can also form
heterodimers. Male and female mice in which the gene for ERα has been knocked
out are sterile, develop osteoporosis, and continue to grow because their
epiphyses do not close. ERβ female knockouts are infertile, but ERβ male
knockouts are fertile even though they have hyperplastic prostates and loss of
fat. Both receptors exist in isoforms and, like thyroid receptors, can bind to
various activating and stimulating factors. In some situations, ERβ can inhibit
ERα transcription. Thus, their actions are complex, multiple, and varied.
Most of the effects of estrogens are genomic, that is, due to actions on
the nucleus, but some are so rapid that it is difficult to believe they are
mediated via production of mRNAs. These include effects on neuronal discharge in
the brain and, possibly, feedback effects on gonadotropin secretion. Evidence
is accumulating that these effects are mediated by cell membrane receptors that
appear to be structurally related to the nuclear receptors and produce their
effects by intracellular mitogen-activated protein kinase pathways. Similar
rapid effects of progesterone, testosterone, glucocorticoids, aldosterone, and
1,25-dihydroxycholecalciferol may also be produced by membrane receptors.
TESTOSTERONE
Chemistry & Biosynthesis of Testosterone
Testosterone, the principal hormone of the testes, is a C19
steroid with a hydroxyl group in the 17 position. It is
synthesized from cholesterol in the Leydig cells and is also formed from
androstenedione secreted by the adrenal cortex. The biosynthetic pathways in all
endocrine organs that form steroid hormones are similar, the organs differing
only in the enzyme systems they contain. In the Leydig cells, the 11- and
21-hydroxylases found in the adrenal cortex are absent, but 17α-hydroxylase is
present. Pregnenolone is therefore hydroxylated in the 17 position and then
subjected to side chain cleavage to form dehydroepiandrosterone.
Androstenedione is also formed via progesterone and 17-hydroxyprogesterone, but
this pathway is less prominent in humans. Dehydroepiandrosterone and
androstenedione are then converted to testosterone.
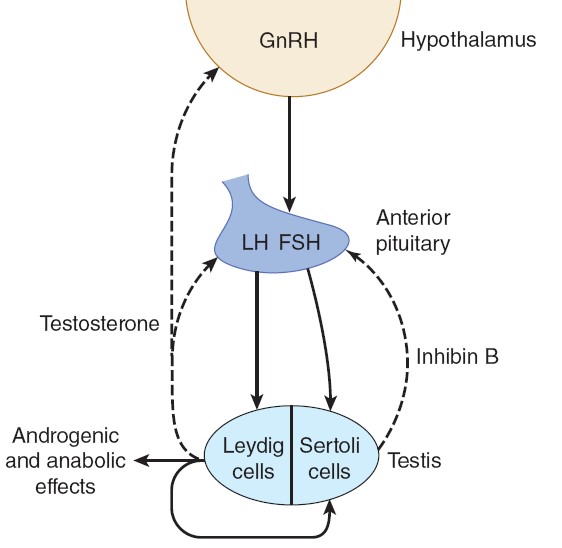
The secretion of testosterone is under the control of LH, and the
mechanism by which LH stimulates Leydig cells involves increased formation of
cyclic adenosine monophosphate (cAMP) via the G-protein–coupled LH receptor and
Gs. Cyclic AMP increases the formation of cholesterol from cholesterol esters
and the conversion of cholesterol to pregnenolone via the activation of protein
kinase A.

Secretion
The testosterone secretion rate is 4–9 mg/d (13.9–31.33 μmol/d) in normal adult
males. Small amounts of testosterone are also secreted in females, with the
major source being the ovary, but possibly from the adrenal as well.
Actions
In addition to their actions during development, testosterone and other
androgens exert an inhibitory feedback effect on pituitary LH secretion; develop
and maintain the male
secondary sex characteristics; exert an important
protein-anabolic, growth-promoting effect; and, along with FSH, maintain
spermatogenesis.
Secondary Sex Characteristics
The widespread changes in hair distribution, body configuration, and genital
size that develop in boys at puberty is called as the male secondary sex
characteristics. The prostate and seminal vesicles enlarge, and the seminal
vesicles begin to secrete fructose. This sugar appears to function as the main
nutritional supply for the spermatozoa. The psychological effects of
testosterone are difficult to define in humans, but in experimental animals,
androgens provoke boisterous and aggressive play. Although body hair is
increased by androgens, scalp hair is decreased. Hereditary baldness often fails
to develop unless dihydrotestosterone (DHT) is present.
Anabolic Effects
Androgens increase the synthesis and decrease the breakdown of protein, leading
to an increase in the rate of growth. It used to be argued that they cause the
epiphyses to fuse to the long bones, thus eventually stopping growth, but it now
appears that epiphysial closure is due to estrogens. Secondary to their anabolic
effects, androgens cause moderate Na+, K+, H2O, Ca2+, SO4–, and PO4– retention;
and they also increase the size of the kidneys. Doses of exogenous testosterone
that exert significant anabolic effects are also masculinizing and increase
libido, which limits the usefulness of the hormone as an anabolic agent in
patients with wasting diseases. Attempts to develop synthetic steroids in which
the anabolic action is separated from the androgenic action have not been
successful.
Mechanism of Action
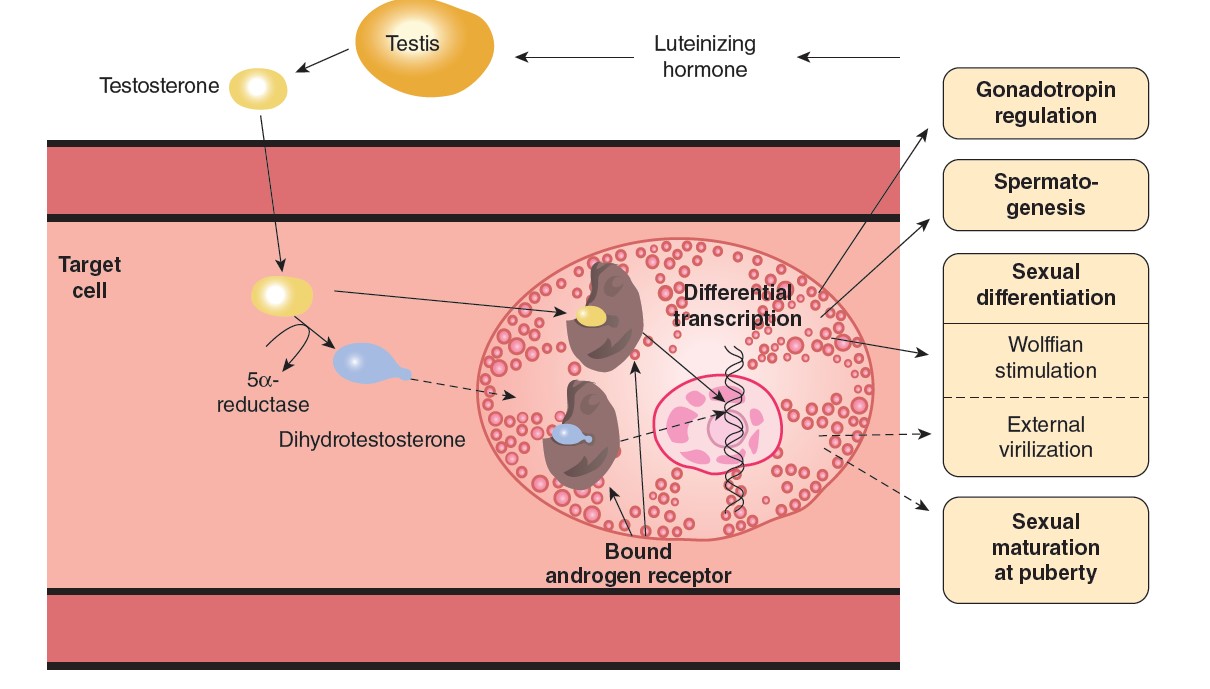
Like other steroids, testosterone binds to an intracellular receptor, and the
receptor/steroid complex then binds to DNA in the nucleus, facilitating
transcription of various genes. In
addition, testosterone is converted to DHT by 5α-reductase in some target
cells, and DHT binds to the same intracellular receptor as testosterone. DHT
also circulates, with a plasma level that is about 10% of the testosterone
level. Testosterone–receptor complexes are less stable than DHT–receptor
complexes in target cells, and they conform less well to the DNA-binding state.
Thus, DHT formation is a way of amplifying the action of testosterone in
target tissues. Humans have two 5α-reductases that are encoded by different
genes. Type 1 5α-reductase is present in skin throughout the body and is the
dominant enzyme in the scalp. Type 2 5α-reductase is present in genital skin,
the prostate, and other genital tissues.
Testosterone–receptor complexes are responsible for the maturation of
Wolffian duct structures and consequently for the formation of male internal
genitalia during development, but DHT–receptor complexes are needed to form
male external genitalia (Figure 23–8). DHT–receptor complexes are also primarily
responsible for enlargement of the prostate and probably of the penis at the
time of puberty, as well as for the facial hair, the acne, and the temporal
recession of the hairline. On the other hand, the increase in muscle mass and
the development of male sex drive and libido depend primarily on testosterone
rather than DHT.
|
Changes at puberty in boys (male secondary sex characteristics). |
|
External genitalia: Penis increases in length and width. Scrotum becomes
pigmented and rugose. |
|
Internal genitalia: Seminal vesicles enlarge and secrete and begin to
form fructose. Prostate and bulbourethral glands enlarge and secrete. |
|
Voice: Larynx enlarges, vocal cords increase in length and thickness,
and voice becomes deeper. |
|
Hair growth: Beard appears. Hairline on scalp recedes anterolaterally.
Pubic hair grows with male (triangle with apex up) pattern. Hair appears
in axillas, on chest, and around anus; general body hair increases. |
|
Mental: More aggressive, active attitude. Interest in opposite sex
develops. |
|
Body conformation: Shoulders broaden; muscles enlarge. |
|
Skin: Sebaceous gland secretion thickens and increases (predisposingto
acne). |
MENOPAUSE
The human ovaries become unresponsive to gonadotropins with advancing age, and
their function declines, so that sexual cycles disappear (menopause).
This unresponsiveness is associated with and probably caused by a decline in the
number of primordial follicles, which becomes precipitous at the time of
menopause
THE
MENSTRUAL CYCLE
The reproductive system of women, unlike that of men, shows regular cyclic
changes that teleologically may be regarded as periodic preparations for
fertilization and pregnancy. In humans and other primates, the cycle is a
menstrual cycle, and its most conspicuous feature is the periodic vaginal
bleeding that occurs with the shedding of the uterine mucosa (menstruation).
The length of the cycle is notoriously variable in women, but an average
figure is 28 days from the start of one menstrual period to the start of the
next. By common usage, the days of the cycle are identified by number, starting
with the first day of menstruation.
Ovarian
Cycle
From the time of birth, there are many primordial follicles under the
ovarian capsule. Each contains an immature ovum. At the start of each cycle,
several of these follicles enlarge, and a cavity forms around the ovum
(antrum formation). This cavity is filled with follicular fluid. In humans,
usually one of the follicles in one ovary starts to grow rapidly on about the
6th day and becomes the dominant follicle, while the others regress,
forming atretic follicles. The atretic process involves apoptosis. It is
uncertain how one follicle is selected to be the dominant follicle in this
follicular phase of the menstrual cycle, but it seems to be related to the
ability of the follicle to secrete the estrogen inside it that is needed for
final maturation. When women are given human pituitary gonadotropin preparations
by injection, many follicles develop simultaneously. The primary source of
circulating estrogen is the granulosa cells of the ovaries; however, the cells
of the theca interna of the follicle are necessary for the production of
estrogen as they secrete androgens that are aromatized to estrogen by the
granulosa cells.
At about the 14th day of the cycle, the distended follicle ruptures, and the
ovum is extruded into the abdominal cavity. This is the process of
ovulation. The ovum is picked up by the fimbriated ends of the uterine tubes
(oviducts). It is transported to the uterus and, unless fertilization occurs,
out through the vagina. The follicle
that ruptures at the time of ovulation promptly fills with blood, forming what
is sometimes called a corpus hemorrhagicum. Minor bleeding from the
follicle into the abdominal cavity may cause peritoneal irritation and fleeting
lower abdominal pain (“mittelschmerz”). The granulosa and theca cells of the
follicle lining promptly begin to proliferate, and the clotted blood is rapidly
replaced with yellowish, lipid-rich luteal cells, forming the corpus
luteum. This initiates the luteal phase of the menstrual cycle,
during which the luteal cells secrete estrogen and progesterone. Growth of the
corpus luteum depends on its developing an adequate blood supply, and there is
evidence that vascular endothelial growth factor (VEGF) is essential for this
process.
If pregnancy occurs, the corpus luteum persists and usually there are no more
periods until after delivery. If pregnancy does not occur, the corpus luteum
begins to degenerate about 4 days before the next menses (24th day of the cycle)
and is eventually replaced by scar tissue, forming a corpus albicans.
The ovarian cycle in other mammals is similar, except that in many
species more than one follicle ovulates and multiple births are the rule.
Corpora lutea form in some submammalian species but not in others. In humans,
no new ova are formed after birth. During fetal development, the ovaries contain
over 7 million primordial follicles. However, many undergo atresia (involution)
before birth and others are lost after birth. At the time of birth, there are 2
million ova, but 50% of these are atretic. The million that are normal undergo
the first part of the first meiotic division at about this time and enter a
stage of arrest in prophase in which those that survive persist until adulthood.
Atresia continues during development, and the number of ova in both of the
ovaries at the time of puberty is less than 300,000. Only one of these ova per
cycle (or about 500 in the course of a normal reproductive life) normally
reaches maturity; the remainder degenerate. Just before ovulation, the first
meiotic division is completed. One of the daughter cells, the secondary
oocyte, receives most of the cytoplasm, while the other, the first polar
body, fragments and disappears. The secondary oocyte immediately begins the
second meiotic division, but this division stops at metaphase and is completed
only when a sperm penetrates the oocyte. At that time, the second polar body
is cast off and the fertilized ovum proceeds to form a new individual. The
arrest in metaphase is due, at least in some species, to formation in the ovum
of the protein pp39mos, which is encoded by the c-mos
protooncogene. When fertilization occurs, the pp39mos is destroyed
within 30 min by calpain, a calcium-dependent cysteine protease.
Uterine Cycle
At the end of menstruation, all but the deep layers of the endometrium have
sloughed. A new endometrium then regrows under the influence of estrogens from
the developing follicle. The endometrium increases rapidly in thickness from the
5th to the 14th days of the menstrual cycle. As the thickness increases, the
uterine glands are drawn out so that they lengthen, but they do not become
convoluted or secrete to any degree. These endometrial changes are called
proliferative, and this part of the menstrual cycle is sometimes called the
proliferative phase. It is also called the preovulatory or follicular phase
of the cycle. After ovulation, the endometrium becomes more highly vascularized
and slightly edematous under the influence of estrogen and progesterone from the
corpus luteum. The glands become coiled and tortuous and they begin to secrete a
clear fluid. Consequently, this phase of the cycle is called the secretory
or luteal phase. Late in the luteal phase, the endometrium, like the
anterior pituitary, produces prolactin, but the function of this endometrial
prolactin is unknown.
The endometrium is supplied by two types of arteries. The superficial two-thirds
of the endometrium that is shed during menstruation, the stratum functionale,
is supplied by long, coiled spiral arteries, whereas the deep layer
that is not shed, the stratum basale, is supplied by short, straight
basilar arteries. When the
corpus luteum regresses, hormonal support for the endometrium is withdrawn. The
endometrium becomes thinner, which adds to the coiling of the spiral arteries.
Foci of necrosis appear in the endometrium, and these coalesce. In addition,
spasm and degeneration of the walls of the spiral arteries take place, leading
to spotty hemorrhages that become confluent and produce the menstrual flow.
The vasospasm is probably produced by locally released prostaglandins.
Large quantities of prostaglandins are present in the secretory endometrium and
in menstrual blood, and infusions of prostagladin F2α(PGF2α) produce endometrial
necrosis and bleeding.
From the point of view of endometrial function, the proliferative phase of the
menstrual cycle represents restoration of the epithelium from the preceding
menstruation, and the secretory phase represents preparation of the uterus for
implantation of the fertilized ovum. The length of the secretory phase is
remarkably constant at about 14 days, and the variations seen in the length of
the menstrual cycle are due for the most part to variations in the length of the
proliferative phase. When fertilization fails to occur during the secretory
phase, the endometrium is shed and a new cycle starts.
Normal Menstruation
Menstrual blood is predominantly arterial, with only 25% of the blood being of
venous origin. It contains tissue debris, prostaglandins, and relatively large
amounts of fibrinolysin from endometrial tissue. The fibrinolysin lyses clots,
so that menstrual blood does not normally contain clots unless the flow is
excessive. The usual duration of the
menstrual flow is 3–5 days, but flows as short as 1 day and as long as 8 days
can occur in normal women. The amount of blood lost may range normally from
slight spotting to 80 mL; the average amount lost is 30 mL. Loss of more than 80
mL is abnormal. Obviously, the amount of flow can be affected by various
factors, including the thickness of the endometrium, medication, and diseases
that affect the clotting mechanism.
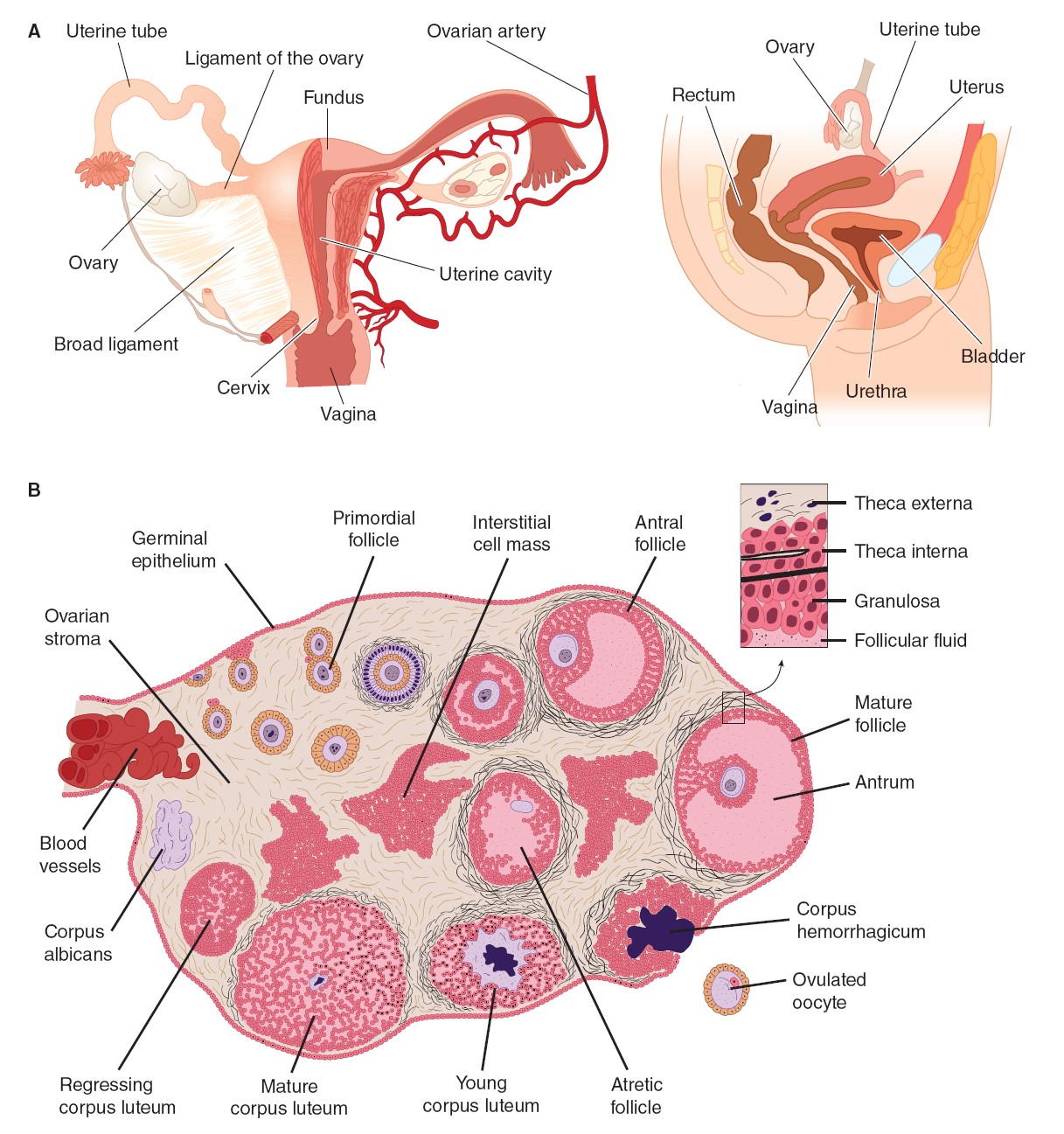
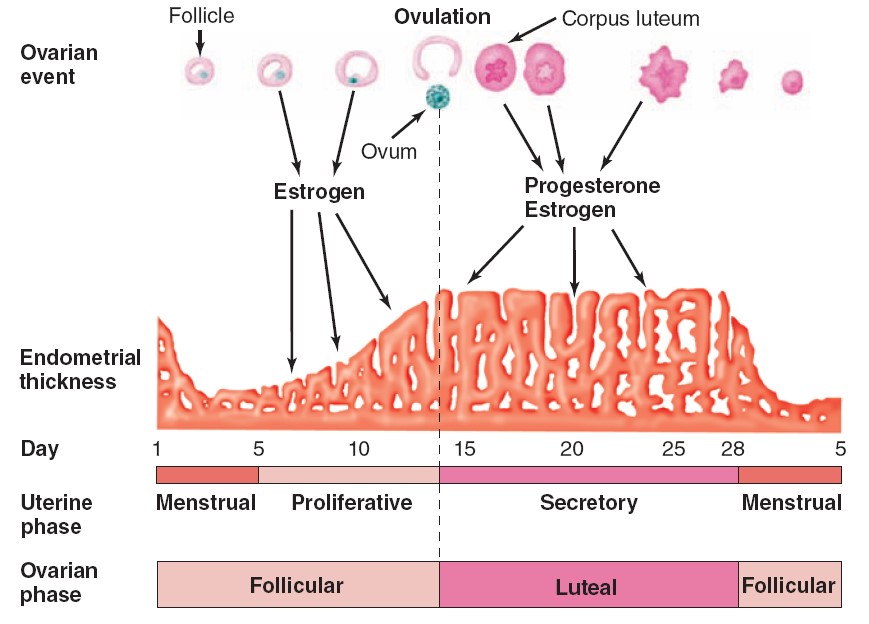
INSULIN
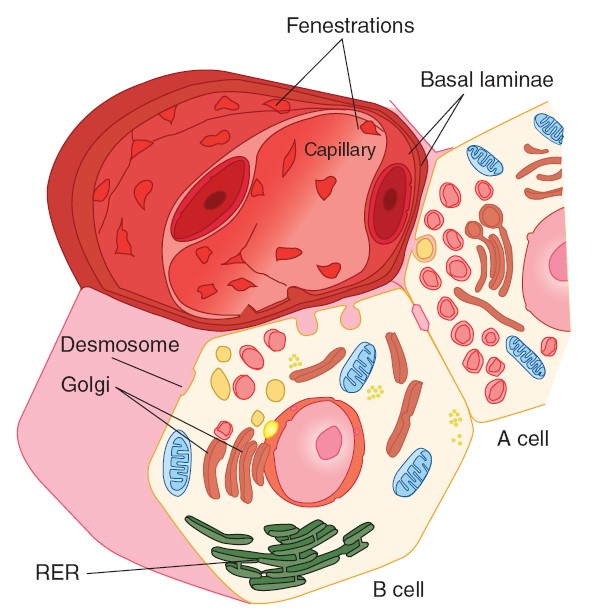
BIOSYNTHESIS AND SECRETION
Insulin is synthesized in the rough endoplasmic reticulum of the B cells. It is
then transported to the Golgi apparatus, where it is packaged into
membrane-bound granules. These granules move to the plasma membrane by a process
involving microtubules, and their contents are expelled by exocytosis. The
insulin then crosses the basal lamina of the B cell and a neighboring capillary
and the fenestrated endothelium of the capillary to reach the bloodstream. Like
other polypeptide hormones and related proteins that enter the endoplasmic
reticulum, insulin is synthesized as part of a larger preprohormone. The gene
for insulin is located on the short arm of chromosome 11 in humans. It has two
introns and three exons. Preproinsulin originates from the endoplasmic
reticulum. The remainder of the molecule is then folded, and the disulfide bonds
are formed to make proinsulin. The peptide segment connecting the A and B
chains, the connecting peptide (C peptide), facilitates the folding and
then is detached in the granules before secretion. Two proteases are involved in
processing the proinsulin. Normally, 90–97% of the product released from the B
cells is insulin along with equimolar amounts of C peptide. The rest is mostly
proinsulin. C peptide can be measured by radioimmunoassay, and its level in
blood provides an index of B cell function in patients receiving exogenous
insulin.
FATE OF SECRETED INSULIN
INSULIN & INSULIN-LIKE ACTIVITY IN BLOOD
Plasma contains a number of substances with insulin-like activity in addition to
insulin. The activity that is not suppressed by anti-insulin antibodies has
been called nonsuppressible insulin-like activity (NSILA). Most, if not
all, of this activity persists after pancreatectomy and is due to the
insulin-like growth factors IGF-I and IGF-II. These IGFs are
polypeptides. Small amounts are free in the plasma (low-molecular-weight
fraction), but large amounts are bound to proteins (high-molecular-weight
fraction). One may well ask why
pancreatectomy causes diabetes mellitus when NSILA persists in the plasma.
However, the insulin-like activities of IGF-I and IGF-II are weak compared to
that of insulin and likely subserve other specific functions.
METABOLISM
The half-life of insulin in the circulation in humans is about 5 min. Insulin
binds to insulin receptors, and some is internalized. It is destroyed by
proteases in the endosomes formed by the endocytotic process.
EFFECTS OF INSULIN
The physiologic effects of insulin are far-reaching and complex. They are
conveniently divided into rapid, intermediate, and delayed actions. The best
known is the hypoglycemic effect, but there are additional effects on amino
acid and electrolyte transport, many enzymes, and growth. The net effect of the
hormone is storage of carbohydrate, protein, and fat. Therefore, insulin is
appropriately called the “hormone of abundance.”
GLUCOSE
TRANSPORTERS
Glucose enters cells by facilitated diffusion or, in the intestine and
kidneys, by secondary active transport with Na+.
In muscle, adipose, and some other tissues, insulin stimulates glucose entry
into cells by increasing the number of glucose transporters (GLUTs) in the cell
membranes.
The GLUTs that are responsible for facilitated diffusion of glucose across cell
membranes are a family of closely related proteins that span the cell membrane
12 times and have their amino and carboxyl terminals inside the cell. They
differ from and have no homology with the sodium-glucose linked transporters
(SGLT-1 and SGLT-2), which are responsible for the secondary active transport of
glucose in the intestine and renal tubules, although the SGLTs also have 12
transmembrane domains. Seven
different GLUTs, named GLUT 1–7 in order of discovery, have been characterized.
They contain 492–524 amino acid residues and their affinity for glucose varies.
Each transporter appears to have evolved for special tasks. GLUT-4 is the
transporter in muscle and adipose tissue that is stimulated by insulin. A pool
of GLUT-4 molecules is maintained within vesicles in the cytoplasm of
insulin-sensitive cells. When the insulin receptors of these cells are
activated, the vesicles move rapidly to the cell membrane and fuse with it,
inserting the transporters into the cell membrane. When insulin action ceases,
the transporter-containing patches of membrane are endocytosed and the vesicles
are ready for the next exposure to insulin. Activation of the insulin receptor
brings about the movement of the vesicles to the cell membrane by activating
phosphatidylinositol 3-kinase. Most of the other GLUT transporters that are not
insulin-sensitive appear to be constitutively expressed in the cell membrane.
In the tissues in which insulin increases the number of GLUTs in cell membranes,
the rate of phosphorylation of the glucose, once it has entered the cells, is
regulated by other hormones. Growth hormone and cortisol both inhibit
phosphorylation in certain tissues. Transport is normally so rapid that it is
not a rate-limiting step in glucose metabolism. However, it is rate-limiting in
B cells. Insulin also increases the
entry of glucose into liver cells, but it does not exert this effect by
increasing the number of GLUT-4 transporters in the cell membranes. Instead, it
induces glucokinase, and this increases the phosphorylation of glucose, so that
the intracellular free glucose concentration stays low, facilitating the entry
of glucose into the cell.
Insulin-sensitive tissues also contain a population of GLUT-4 vesicles that move
into the cell membrane in response to exercise, a process that occurs
independent of the action of insulin. This is why exercise lowers blood sugar. A
5’-adenosine monophosphate (AMP)–activated kinase may trigger the insertion of
these vesicles into the cell membrane.
RELATION TO POTASSIUM
Insulin causes K+ to enter cells, with a resultant lowering of the extracellular
K+ concentration. Infusions of insulin and glucose significantly lower the
plasma K+ level in normal individuals and are very effective for the temporary
relief of hyperkalemia in patients with renal failure. Hypokalemia often
develops when patients with diabetic acidosis are treated with insulin. The
reason for the intracellular migration of K+ is still uncertain. However,
insulin increases the activity of Na, K ATPase in cell membranes, so that more
K+ is pumped into cells.
OTHER ACTIONS
The action on glycogen synthase fosters glycogen storage, and the actions on
glycolytic enzymes favor glucose metabolism to two carbon fragments, with
resulting promotion of lipogenesis. Stimulation of protein synthesis from amino
acids entering the cells and inhibition of protein degradation foster growth.
The anabolic effect of insulin is aided by the protein-sparing action of
adequate intracellular glucose supplies. Failure to grow is a symptom of
diabetes in children, and insulin stimulates the growth of immature
hypophysectomized rats to almost the same degree as growth hormone.
MECHANISM OF ACTION
INSULIN
RECEPTORS
Insulin receptors are found on many different cells in the body, including cells
in which insulin does not increase glucose uptake.
The insulin receptor, which has a molecular weight of approximately
340,000, is a tetramer made up of two α and two β glycoprotein subunits. All
these are synthesized on a single mRNA and then proteolytically separated and
bound to each other by disulfide bonds. The gene for the insulin receptor has 22
exons and in humans is located on chromosome 19. The α subunits bind insulin and
are extracellular, whereas the β subunits span the membrane. The intracellular
portions of the β subunits have tyrosine kinase activity. The α and β subunits
are both glycosylated, with sugar residues extending into the interstitial
fluid. Binding of insulin triggers
the tyrosine kinase activity of the β subunits, producing autophosphorylation of
the β subunits on tyrosine residues. The autophosphorylation, which is necessary
for insulin to exert its biologic effects, triggers phosphorylation of some
cytoplasmic proteins and dephosphorylation of others, mostly on serine and
threonine residues. Insulin receptor substrate (IRS-1) mediates some of the
effects in humans but there are other effector systems as well. For example,
mice in which the insulin receptor gene is knocked out show marked growth
retardation in utero, have abnormalities of the central nervous system (CNS)
and skin, and die at birth of respiratory failure, whereas IRS-1 knockouts show
only moderate growth retardation in utero, survive, and are insulin-resistant
but otherwise nearly normal. The
growth-promoting protein anabolic effects of insulin are mediated via
phosphatidylinositol 3-kinase (PI3K), and evidence indicates that in
invertebrates, this pathway is involved in the growth of nerve cells and axon
guidance in the visual system. It is
interesting to compare the insulin receptor with other related receptors. The
insulin receptor is very similar to the receptor for IGF-I but different from
the receptor for IGF-II. Other receptors for growth factors and receptors for
various oncogenes also are tyrosine kinases. However, the amino acid
composition of these receptors is quite different.
When insulin binds to its receptors, they aggregate in patches and are
taken up into the cell by receptor-mediated endocytosis. Eventually, the
insulin–receptor complexes enter lysosomes, where the receptors are broken down
or recycled. The half-life of the insulin receptor is about 7 h.
|
Principal actions of insulin. |
|
Rapid (seconds) |
|
Increased transport of glucose, amino acids, and K+ into
insulin-sensitive cells |
|
Intermediate (minutes) |
|
Stimulation of protein synthesis |
|
Inhibition of protein degradation |
|
Activation of glycolytic enzymes and glycogen synthase |
|
Inhibition of phosphorylase and gluconeogenic enzymes |
|
Delayed (hours) |
|
Increase in mRNAs for lipogenic and other enzymes |
|
Effects of insulin on various tissues. |
|
Adipose tissue
Increased glucose entry
Increased fatty acid synthesis
Increased glycerol phosphate synthesis
Increased triglyceride deposition
Activation of lipoprotein lipase
Inhibition of hormone-sensitive lipase
Increased K+uptake |
|
Muscle
Increased glucose entry
Increased glycogen synthesis
Increased amino acid uptake
Increased protein synthesis in ribosomes
Decreased protein catabolism
Decreased release of gluconeogenic amino acids
Increased ketone uptake
Increased K+uptake |
|
Liver
Decreased ketogenesis
Increased protein synthesis
Increased lipid synthesis
Decreased glucose output due to decreased gluconeogenesis, increased
glycogen synthesis, and increased glycolysis |
|
General
Increased cell growth |
Reference Books:
1. Ganong’s Review of Medical Physiology, 25edition, 2016.
2. Guyton_Hall_Textbook_of_Medical_Physiology, 13th edition, 2016.
3. Vander’s Human Physiology, 14th edition, 2015.
4. Essentials of Medical Physiology, 6th edition, K Sembulingam.
5. Fox_S._Human_physiology, 12th edition.
6. Hansen KoeppenNetter's Atlas of Human Physiology, Saunders, 2002.
7.
Krieger_A_Visual_Analogy_Guide_to_Human_Anatomy_&_Physiology
8.
Lauralee_Sherwood_Introduction_to_Human_Physiology 8th edition.
9.
RoddieWallace_MCQs_and_EMQs_in_Human_Physiology 6th edition.