DIGESTIVE SYSEM
OVERVIEW:

The digestive system includes the gastrointestinal (GI) tract (or
alimentary canal), consisting of the mouth, pharynx, esophagus, stomach,
small intestine, and large intestine; and the accessory organs and tissues,
consisting of the salivary glands, liver, gallbladder, and exocrine pancreas.
The accessory organs are not part of the tract but secrete substances into it
via connecting ducts. The overall function of the digestive system is to process
ingested foods into molecular forms that are then transferred, along with small
molecules, ions, and water, to the body’s internal environment, where the
circulatory system can distribute them to cells. The digestive system is under
the local neural control of the enteric nervous system and also of the central
nervous system. The adult gastrointestinal tract is a tube approximately 9 m (30
feet) in length, running through the body from mouth to anus. The lumen
of the tract is continuous with the external environment, which means that its
contents are technically outside the body. This fact is relevant to
understanding some of the tract’s properties. For example, the large intestine
is colonized by billions of bacteria, most of which are harmless and even
beneficial in this location. However, if the same bacteria enter the internal
environment, as may happen, for example, if a portion of the large intestine is
perforated, they may cause a severe infection.
Most food enters the gastrointestinal tract as large particles containing
macromolecules, such as proteins and polysaccharides, which are unable to cross
the intestinal epithelium. Before ingested food can be absorbed, therefore, it
must be dissolved and broken down into small molecules. (Small nutrients such as
vitamins and minerals do not need to be broken down and can cross the epithelium
intact.) This dissolving and breaking-down process is called digestion
and is accomplished by the action of hydrochloric acid in the stomach, bile from
the liver, and a variety of digestive enzymes released by the system’s exocrine
glands. Each of these substances is released into the lumen of the GI tract
through the process of secretion. In addition, some digestive enzymes are
located on the apical membranes of the intestinal epithelium. The molecules
produced by digestion, along with water and small nutrients that do not require
digestion, then move from the lumen of the gastrointestinal tract across a layer
of epithelial cells and enter the blood or lymph. This process is called
absorption.
While digestion, secretion, and absorption are taking place, contractions of
smooth muscles in the gastrointestinal tract wall occur, where they serve two
functions: They mix the luminal contents with the various secretions, and they
move the contents through the tract from mouth to anus. These contractions are
referred to as the motility of the gastrointestinal tract. In some cases,
muscular movements travel in a wavelike fashion in one direction along the
length of a part of the tract, a process called peristalsis. The
functions of the digestive system can be described in terms of these four major
processes—digestion, secretion, absorption, and motility—and
the mechanisms controlling them. Within fairly wide limits, the digestive system
will absorb as much of any particular substance that is ingested, with a few
important exceptions (to be described later). Therefore, the digestive system
does not regulate the total amount of nutrients absorbed or their concentrations
in the internal environment. The plasma concentration and distribution of the
absorbed nutrients throughout the body are primarily controlled by hormones from
a number of endocrine glands and by the kidneys. Small amounts of certain
metabolic end products are excreted via the gastrointestinal tract, primarily by
way of the bile. This represents a relatively minor function of the GI tract in
healthy individuals—elimination. In fact, the lungs and kidneys are
usually responsible for the elimination of most of the body’s waste products,
such as CO2. The material known as feces leaves the system via the anus
at the end of the gastrointestinal tract. Feces consist almost entirely of
bacteria and ingested material that was neither digested nor absorbed,
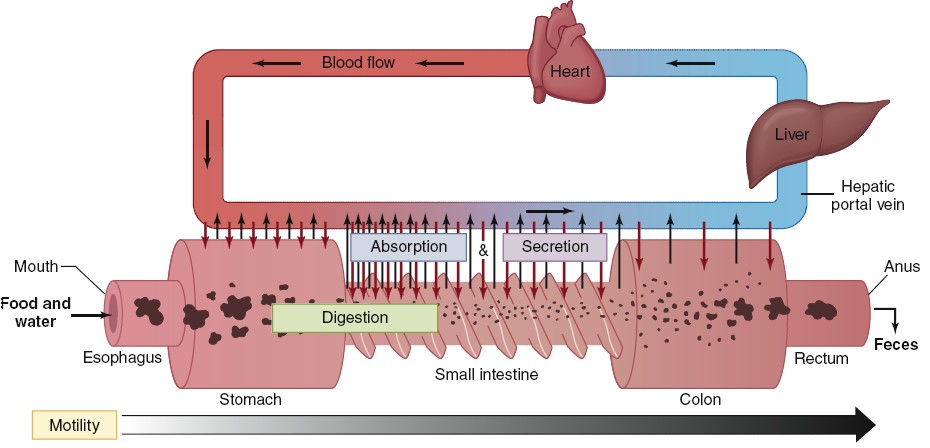
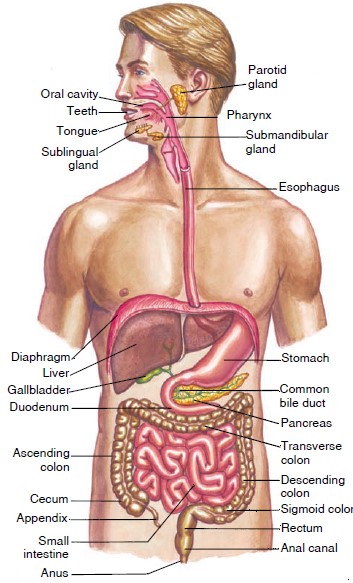
Four major processes the gastrointestinal tract carries out: digestion,
secretion, absorption, and motility. Outward-pointing (black) arrows indicate
absorption of the products of digestion, water, minerals, and vitamins into the
blood. Inward-pointing (red) arrows represent the secretion of ions, enzymes,
and bile salts into the GI tract. The length and density of the arrows indicate
the relative importance of each segment of the tract; the small intestine is
where most digestion, absorption, and secretion occurs. The feces represent a
fifth function of the GI tract: elimination. The wavy configuration of the small
intestine represents muscular contractions (motility) throughout the tract.
Functions of the Organs of the Digestive System
|
Organ
|
Exocrine Secretions
|
Functions Related to Digestion and Absorption
|
|
Mouth and pharynx |
|
Chewing begins; initiation of swallowing reflex |
|
Salivary glands |
Ions and water |
Moisten and dissolve food; help neutralize ingested acid |
|
|
Mucus |
Lubrication |
|
|
Amylase |
Polysaccharide-digesting enzyme (relatively minor function) |
|
|
Antibodies and other immune factors |
Help prevent tooth and gum decay |
|
Esophagus |
|
Move food to stomach by peristaltic waves |
|
|
Mucus |
Lubrication |
|
Stomach |
|
Store, mix, dissolve, and continue digestion of food; regulate
emptying of dissolved food into small intestine |
|
|
HCl |
Solubilization of some food particles; kill microbes;
activation of pepsinogen to pepsin |
|
|
Pepsin |
Begin the process of protein digestion in the stomach |
|
|
Mucus |
Lubricate and protect epithelial surface |
|
Pancreas |
|
Secretion of enzymes and bicarbonate; also has nondigestive
endocrine functions |
|
|
Enzymes |
Digest carbohydrates, fats, proteins, and nucleic acids |
|
|
Bicarbonate |
Neutralize HCl entering small intestine from stomach |
|
Liver |
|
Secretion of bile |
|
|
Bile salts |
Solubilize water-insoluble fats |
|
|
Bicarbonate |
Neutralize HCl entering small intestine from stomach |
|
|
Organic waste products and trace metals |
Elimination in feces |
|
Gallbladder |
|
Store and concentrate bile between meals |
|
Small intestine |
|
Digestion and absorption of most substances; mixing and
propulsion of contents |
|
|
Enzymes |
Digestion of macromolecules |
|
|
Ions and water |
Maintain fluidity of luminal contents |
|
|
Mucus |
Lubrication and protection |
|
Large intestine |
|
Storage and concentration of undigested matter; absorption of
ions and water; mixing and propulsion of contents; defecation |
|
|
Mucus |
Lubrication |
Defecation
After electrolytes and water have been absorbed the waste material that is left
passes to the rectum, leading to an increase in rectal pressure, relaxation of
the internal anal sphincter, and the urge to defecate. If the urge to defecate
is denied, feces are prevented from entering the anal canal by the external anal
sphincter. In this case, the feces remain in the rectum, and may even back up
into the sigmoid colon. The defecation reflex normally occurs when the rectal
pressure rises to a particular level that is determined, to a large degree, by
habit. At this point the external anal sphincter relaxes to admit feces into the
anal canal. During the act of defecation, the longitudinal rectal muscles
contract to increase rectal pressure, and the internal and external anal
sphincter muscles relax. Excretion is aided by contractions of abdominal and
pelvic skeletal muscles, which raise the intra-abdominal pressure. The raised
pressure helps push the feces from the rectum, through the anal canal, and out
of the anus.
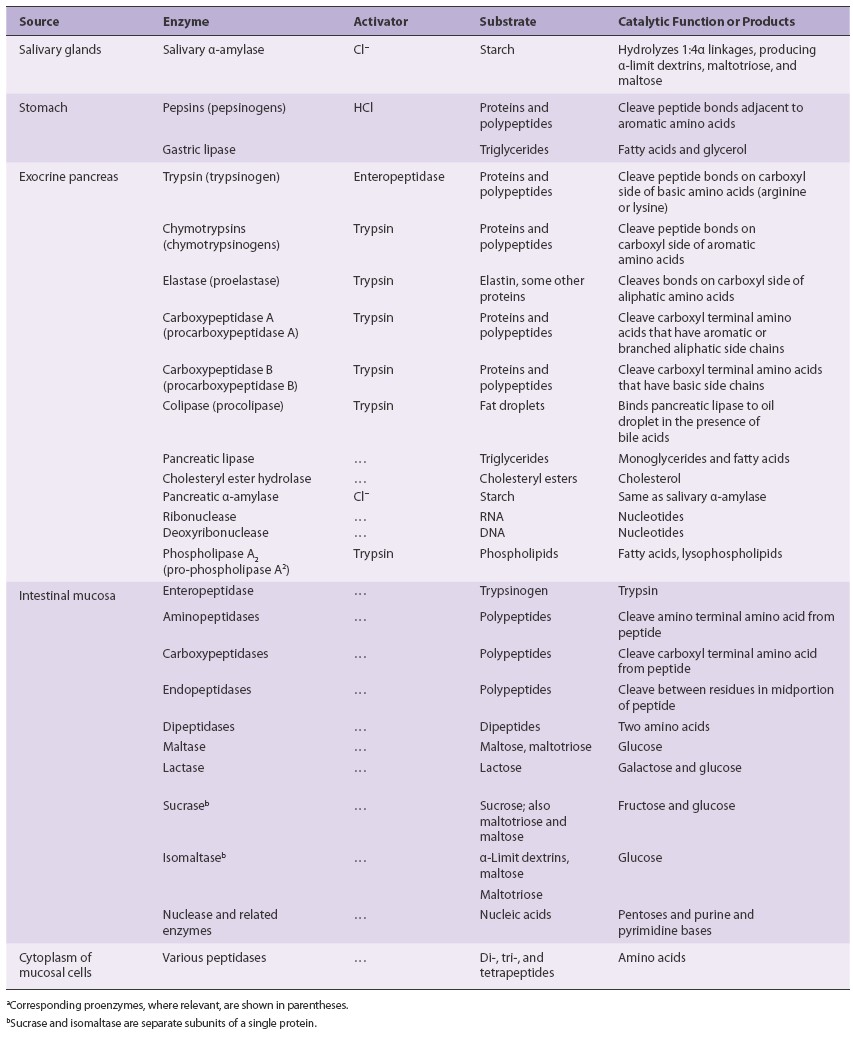
Major digestive
enzymes:
|
Enzyme
|
Site of Action |
Source
|
Substrate
|
Optimum pH |
Product(s)
|
|
Salivary amylase |
Mouth
|
Saliva
|
Starch
|
6.7
|
Maltose
|
|
Pepsin
|
Stomach
|
Gastric glands |
Protein
|
1.6–2.4
|
Shorter polypeptides |
|
Pancreatic amylase |
Duodenum
|
Pancreatic juice |
Starch
|
6.7–7.0
|
Maltose, maltriose, and oligosaccharides |
|
Trypsin, chymotrypsin, carboxypeptidase |
Small intestine |
Pancreatic juice |
Polypeptides
|
8.0
|
Amino acids, dipeptides, and tripeptides |
|
Pancreatic lipase |
Small intestine |
Pancreatic juice |
Triglycerides
|
8.0
|
Fatty acids and monoglycerides |
|
Maltase
|
Small intestine |
Brush border of epithelial cells |
Maltose
|
5.0–7.0
|
Glucose
|
|
Sucrase
|
Small intestine |
Brush border of epithelial cells |
Sucrose
|
5.0–7.0
|
Glucose + fructose |
|
Lactase
|
Small intestine |
Brush border of epithelial cells |
Lactose
|
5.8–6.2
|
Glucose + galactose |
|
Aminopeptidase
|
Small intestine |
Brush border of epithelial cells |
Polypeptides
|
8.0
|
Amino acids, dipeptides, tripeptides |
Enzymes of
pancreas:
|
Enzyme
|
Zymogen
|
Activator
|
Action
|
|
Trypsin
|
Trypsinogen
|
Enterokinase
|
Cleaves internal peptide bonds |
|
Chymotrypsin
|
Chymotrypsinogen
|
Trypsin
|
Cleaves internal peptide bonds |
|
Elastase
|
Proelastase
|
Trypsin
|
Cleaves internal peptide bonds |
|
Carboxypeptidase
|
Procarboxypeptidase
|
Trypsin
|
Cleaves last amino acid from carboxyl-terminal end of polypeptide |
|
Phospholipase
|
Prophospholipase
|
Trypsin
|
Cleaves fatty acids from phospholipids such as lecithin |
|
Lipase
|
None
|
None
|
Cleaves fatty acids from glycerol |
|
Amylase
|
None
|
None
|
Digests starch to maltose and short chains of glucose molecules |
|
Cholesterolesterase
|
None
|
None
|
Releases cholesterol from its bonds with other molecules |
|
Ribonuclease
|
None
|
None
|
Cleaves RNA to form short chains |
|
Deoxyribonuclease
|
None
|
None
|
Cleaves DNA to form short chains |
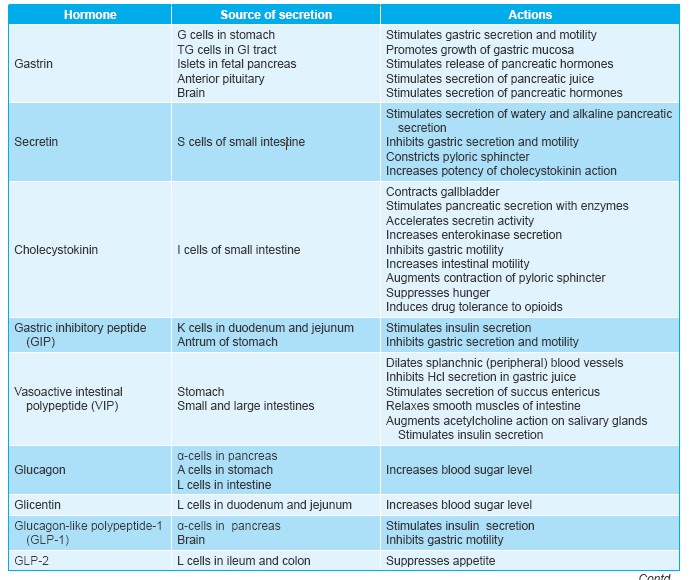
Effects of Gastric Hormones:
|
Secreted by |
Hormone
|
Effects
|
|
Stomach
|
Gastrin
|
Stimulates parietal cells to secrete HCl |
|
|
|
Stimulates chief cells to secrete pepsinogen |
|
|
|
Maintains structure of gastric mucosa |
|
Small intestine |
Secretin
|
Stimulates water and bicarbonate secretion in pancreatic juice |
|
|
|
Potentiates actions of cholecystokinin on pancreas |
|
Small intestine |
Cholecystokinin (CCK) |
Stimulates contraction of gallbladder |
|
|
|
Stimulates secretion of pancreatic juice enzymes |
|
|
|
Inhibits gastric motility and secretion Maintains structure of exocrine
pancreas (acini) |
|
Small intestine |
Gastric inhibitory peptide (GIP) |
Inhibits gastric motility and secretion |
|
|
|
Stimulates secretion of insulin from pancreatic islets |
|
Ileum and colon |
Glucagon-like peptide-I (GLP-I) |
Inhibits gastric motility and secretion |
|
|
|
Stimulates secretion of insulin from pancreatic islets |
|
|
Guanylin
|
Stimulates intestinal secretion of Cl−, causing elimination of NaCl and
water in the feces |
Phases of Gastrointestinal Control:
The neural and hormonal control of the digestive system is, in large part,
divisible into three phases—cephalic, gastric, and intestinal—according towhere
the stimulus is perceived.
The cephalic (from a Greek word for “head”) phase is initiated
when sensory receptors in the head are stimulated by sight, smell, taste, and
chewing. Various emotional states can also initiate this phase. The efferent
pathways for these reflexes are primarily mediated by parasympathetic fibers
carried in the vagus nerves. These fibers activate neurons in the
gastrointestinal nerve plexuses, which in turn affect secretory and contractile
activity.
Four stimuli in the stomach initiate the reflexes that constitute the gastric
phase of regulation: distension, acidity, amino acids, and peptides formed
during the partial digestion of ingested
protein. The
responses to these stimuli are mediated by short and long neural reflexes and by
release of the hormone gastrin.
Finally, the intestinal phase is initiated by stimuli in the small
intestine including distension, acidity, osmolarity, and various digestive
products. The intestinal phase is mediated by both short and long neural
reflexes and by the hormones secretin, CCK, and GIP, all of which are secreted
by enteroendocrine cells of the small intestine. Each of these phases is named
for the site at which the various stimuli initiate the reflex and not for the
sites of effector activity. Each phase is characterized by efferent output to
virtually all organs in the gastrointestinal tract. Also, these phases do not
occur in temporal sequence except at the very beginning of a meal. Rather,
during ingestion and the much longer absorptive period, reflexes characteristic
of all three phases may be occurring simultaneously.
Digestion and Absorption of carbohydrates
The average daily intake of carbohydrates is about 250 to 300 g per day in a
typical American diet. This represents about half the average daily intake of
calories. About two-thirds of this carbohydrate is the plant polysaccharide
starch, and most of the remainder consists of the disaccharides sucrose (table
sugar) and lactose
(milk sugar). Only small amounts of monosaccharides are normally present in
the diet. Cellulose and certain other complex polysaccharides found in vegetable
matter—referred to as dietary fiber (or simply fiber)—are not broken down
by the enzymes in the small intestine and pass on to the large intestine, where
they are partially metabolized by bacteria. The digestion of starch by salivary
amylase begins in the mouth but accounts for only a small fraction of total
starch digestion. It continues very briefly in the upper part of the stomach
before gastric acid inactivates the amylase. Most (~95% or more) starch
digestion is completed in the small intestine by pancreatic amylase.
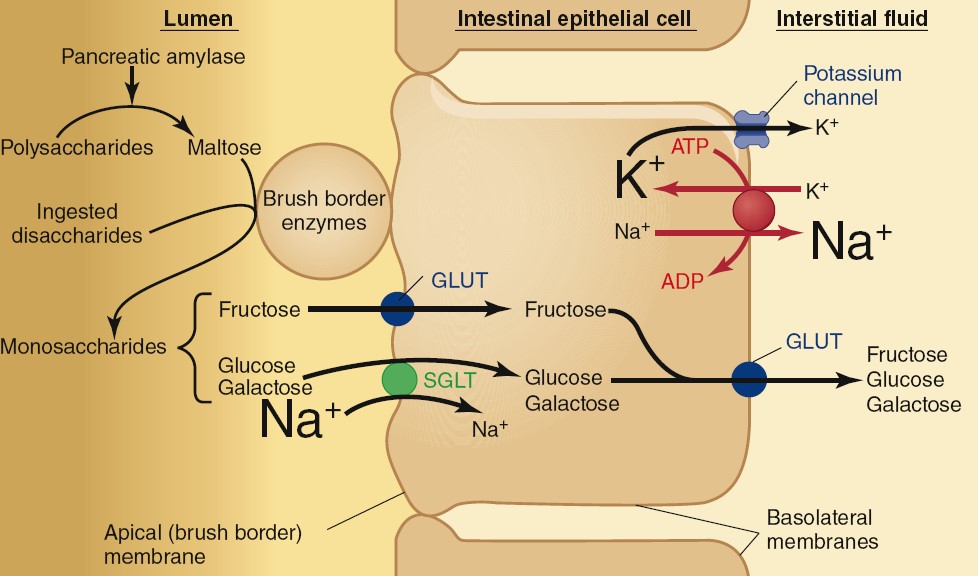
The products of both salivary and pancreatic amylase are the disaccharide
maltose and a mixture of short, branched chains of glucose molecules. These
products, along with ingested sucrose and lactose, are broken down into
monosaccharides—glucose, galactose, and fructose—by enzymes located on the
apical membranes of the small-intestine epithelial cells (brush border). These
monosaccharides are then transported across the intestinal epithelium into the
blood. Fructose enters the epithelial cells by facilitated diffusion via a
glucose transporter (GLUT), whereas glucose and galactose undergo secondary
active transport coupled to Na1 via the sodium–glucose cotransporter (SGLT).
These monosaccharides then leave the epithelial cells and enter the interstitial
fluid by way of facilitated diffusion via various GLUT proteins in the
basolateral membranes of the epithelial cells. From there, the monosaccharides
diffuse into the blood through capillary pores. Most ingested carbohydrates are
digested and absorbed within the first 20% of the small intestine.
Digestion and Absorption of proteins
A healthy adult requires a minimum of about 40 to 50 g of protein per day to
supply essential amino acids and replace the nitrogen contained in amino acids
that are metabolized to urea. A typical American diet contains about 60 to 90 g
of protein per day. This represents about one-sixth of the average daily caloric
intake. In addition, a large amount of protein, in the form of enzymes and
mucus, is secreted into the GI tract or enters it via the death and
disintegration of epithelial cells. Regardless of the source, most of the
protein in the lumen is broken down into dipeptides, tripeptides, and amino
acids, all of which are absorbed by the small intestine.
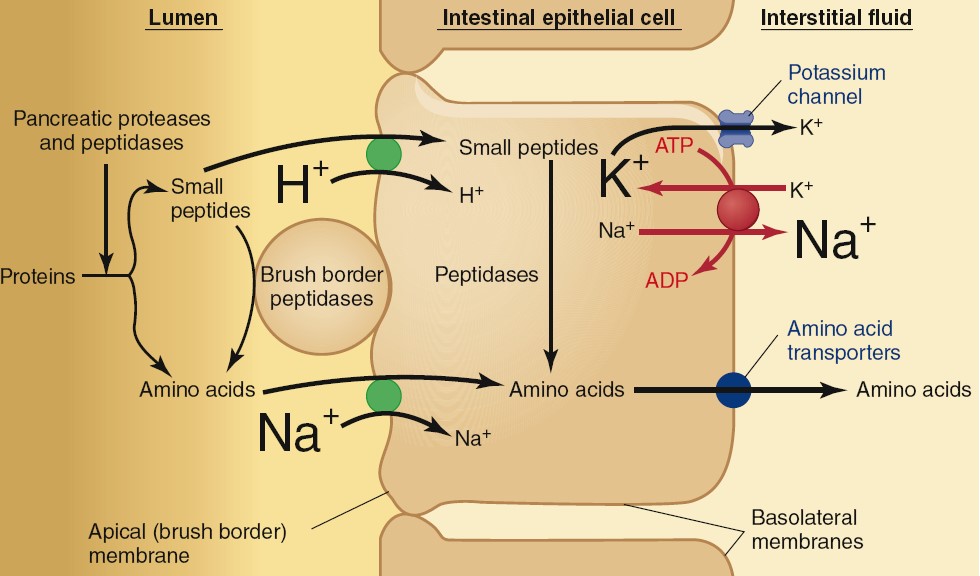
Proteins are first partially broken down to peptide fragments in the stomach by
the enzyme pepsin that is produced from an inactive precursor
pepsinogen. Further breakdown is completed in the small intestine by the
enzymes trypsin and chymotrypsin, the major proteases secreted by
the pancreas. These peptide fragments can be absorbed if they are small enough
or are further digested to free amino acids by carboxypeptidases
(additional proteases secreted by the pancreas) and aminopeptidases,
located on the apical membranes of the small-intestine epithelial cells. These
last two enzymes split off amino acids from the carboxyl and amino ends of
peptide fragments, respectively. At least 20 different peptidases are located on
the apical membrane of the epithelial cells, with various specificities for the
peptide bonds they attack. Most of the products of protein digestion are
absorbed as short chains of two or three amino acids by secondary active
transport coupled to the H+ gradient. The absorption of small
peptides contrasts with carbohydrate absorption, in which molecules larger than
monosaccharides are not absorbed. Free amino acids, by contrast, enter the
epithelial cells by secondary active transport coupled to Na+. There
are many different amino acid transporters that are specific for the different
amino acids, but only one transporter for simplicity. Within the cytosol of the
epithelial cell, the dipeptides and tripeptides are hydrolyzed to amino acids;
these, along with free amino acids that entered the cells, then leave the cell
and enter the interstitial fluid through facilitated-diffusion transporters in
the basolateral membranes. As with carbohydrates, protein digestion and
absorption are largely completed in the upper portion of the small intestine.
Very small amounts of intact proteins are able to cross the intestinal
epithelium and gain access to the interstitial fluid. They do so by a
combination of endocytosis and exocytosis. The absorptive capacity for intact
proteins is much greater in infants
than in adults, and antibodies (proteins involved in the immunologic defense
system of the body) secreted into the mother’s milk can be absorbed intact by
the infant, providing some immunity until the infant’s immune system matures.
Digestion and Absorption of fats
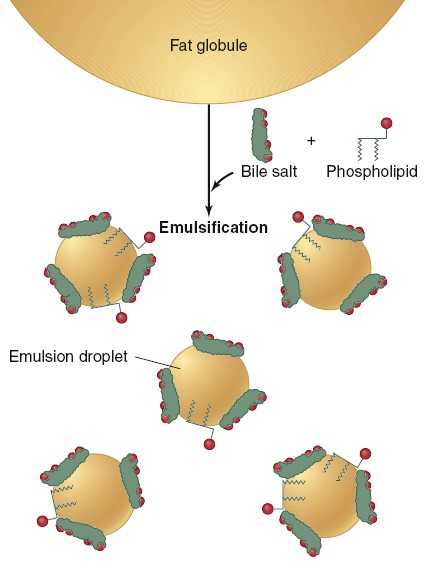
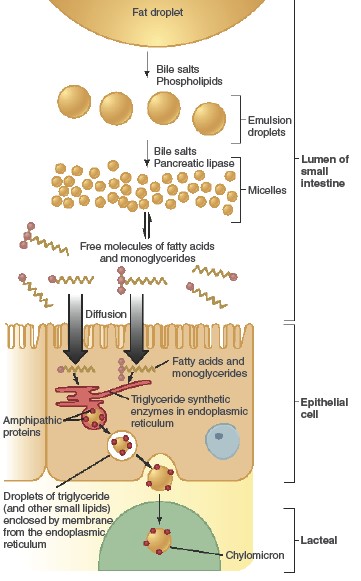
Emulsification of fat by bile salts and phospholipids. Note that the nonpolar
sides (green) of bile salts and phospholipids are oriented toward fat, whereas
the polar sides (red) of these compounds are oriented outward.
Digestion
Triglyceride digestion occurs to a limited extent in the mouth and stomach, but
it predominantly occurs in the small intestine. The major digestive enzyme in
this process is pancreatic lipase, which catalyzes the splitting of bonds
linking fatty acids to the first and third carbon atoms of glycerol, producing
two free fatty acids and a monoglyceride as products:
Emulsification
The lipids in the ingested foods are insoluble in water and aggregate into large
lipid droplets in the upper portion of the stomach. This is like a mixture of
oil and vinegar after shaking. Because pancreatic lipase is a water soluble
enzyme, its digestive action in the small intestine can take place only at the
surface of a lipid droplet. Therefore, if most of the ingested fat
remained in large lipid droplets, the rate of triglyceride digestion would be
very slow because of the small surface-area-to-volume ratio of these big fat
droplets. The rate of digestion is, however, substantially increased by division
of the large lipid droplets into many very small droplets, each about 1 mm in
diameter, thereby increasing their surface area and accessibility to lipase
action. This process is known as emulsification, and the resulting
suspension of small lipid droplets is called an emulsion.
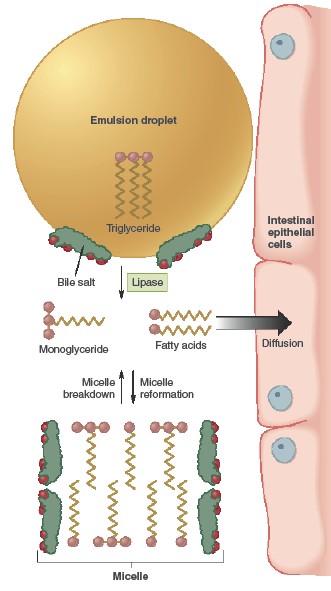
The emulsification of fat requires (1)
mechanical disruption of the large lipid droplets into smaller droplets and (2)
an emulsifying agent, which acts to prevent the smaller droplets from
reaggregating back into large droplets. The mechanical disruption is provided by
the motility of the GI tract, occurring in the lower portion of the stomach and
in the small intestine, which grinds and mixes the luminal contents.
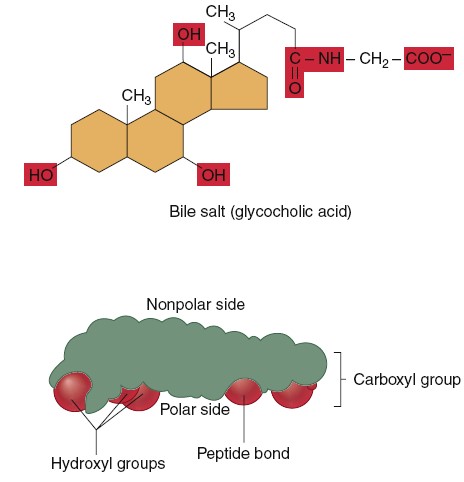
Phospholipids in food, along with phospholipids and bile salts secreted in the
bile, provide the emulsifying
agents. Phospholipids are amphipathic molecules consisting of two nonpolar fatty
acid chains attached to glycerol, with a charged phosphate group located on
glycerol’s third carbon. Bile salts are formed from cholesterol in the liver and
are also amphipathic. The
nonpolar portions of the phospholipids and bile salts associate with the
nonpolar interior of the lipid droplets, leaving the polar portions exposed at
the water surface. There, they repel other lipid droplets that are similarly
coated with these emulsifying agents, thereby preventing their reaggregation
into larger fat droplets. The coating of the lipid droplets with these
emulsifying agents, however, impairs the accessibility of the water-soluble
pancreatic lipase to its lipid substrate. To overcome this problem, the pancreas
secretes a protein known as colipase, which is amphipathic and lodges on
the lipid droplet surface. Colipase binds the lipase enzyme, holding it on the
surface of the lipid droplet.
Absorption
Although emulsification speeds up digestion, absorption of the water-insoluble
products of the lipase reaction would still be very slow if it were not for a
second action of the bile salts, the formation of micelles, which are
similar in structure to emulsion droplets but much smaller—4 to 7 nm in
diameter. Micelles consist of bile salts, fatty acids, monoglycerides, and
phospholipids all clustered together with the polar ends of each molecule
oriented toward the micelle’s surface and the nonpolar portions forming the
micelle’s core. Also included in the core of the micelle are small amounts of
fat-soluble vitamins and cholesterol.
The average daily intake of lipids is 70 to 100 g per day in a typical American
diet, most of this in the form of fat (triglycerides). This represents about
one-third of the average daily caloric intake.
HCL PRODUCTION AND
SECRETION
The stomach secretes about 2 L of hydrochloric acid per day. The concentration
of H+ in the lumen of the stomach may reach >150 mM, which is 1 to 3
million times greater than the concentration in the blood. This requires an
efficient production mechanism to generate large numbers of hydrogen ions. The
origin of the hydrogen ions is CO2 in the parietal cell, which
contains the enzyme carbonic anhydrase.
Carbonic anhydrase catalyzes the reaction between CO2 with water to
produce carbonic acid, which dissociates to H+ and HCO3-.
Primary H+/K+-ATPases in the apical membrane of the
parietal cells pump these hydrogen ions into the lumen of the stomach. This
primary active transporter also pumps K+ into the cell, which then
leaks back into the lumen through K+ channels. As H+ is
secreted into the lumen, HCO3- is secreted on the opposite side of
the cell in exchange for Cl-,
which maintains electroneutrality. Removal of the end products (H+
and HCO3-) of this reaction enhances the rate of the reaction by the
law of mass action. In this way, production and secretion of H+ are
coupled. Increased acid secretion results from the transfer of H+/K+-ATPase
proteins from the membranes of intracellular vesicles to the plasma membrane by
fusion of these vesicles with the apical membrane, thereby increasing the number
of pump proteins in the apical plasma membrane. This process is analogous to the
transfer of water channels (aquaporins) to the apical plasma membrane of kidney
collecting-duct cells in response to ADH.
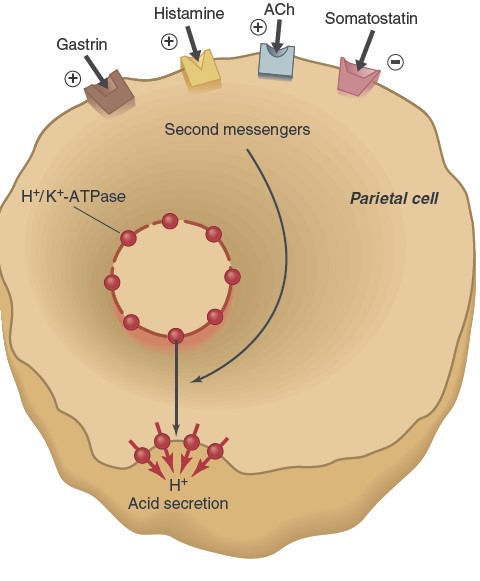
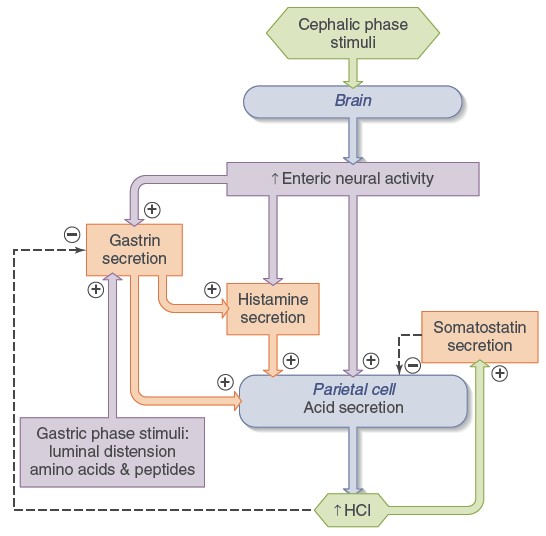
Three chemical messengers stimulate the insertion of H+/K+-ATPases
into the plasma membrane and therefore acid secretion: gastrin (a gastric
hormone), acetylcholine (ACh, a neurotransmitter), and histamine (a paracrine
substance). By contrast, somatostatin—another paracrine substance—inhibits
acid secretion. Parietal cell membranes contain receptors for all four of
these molecules. This illustrates the general principle of physiology that most
physiological functions—in this case, the secretion of H+ into the
stomach lumen—are controlled by multiple regulatory systems, often working in
opposition. These chemical messengers not only act directly on the parietal
cells but also influence each other’s secretion. For example, histamine markedly
potentiates the response to the other two stimuli, gastrin and ACh, and gastrin
and ACh both stimulate histamine secretion. During a meal, the rate of acid
secretion increases markedly as stimuli arising from the cephalic, gastric, and
intestinal phases alter the release of the four chemical messengers described in
the previous paragraph. During the cephalic phase, increased activity of
efferent parasympathetic neural input to the stomach’s enteric nervous system
results in the release of ACh from the plexus neurons, gastrin from the
gastrin-releasing G cells, and histamine from ECL cells.
Once food has reached the stomach, the gastric phase stimuli—distension from the
volume of ingested material and the presence of peptides and amino acids
released by the digestion of luminal proteins—produce a further increase in acid
secretion. These stimuli use some of the same neural pathways used during the
cephalic phase. Neurons in the mucosa of the stomach respond to these luminal
stimuli and send action potentials to the cells of the enteric nervous system,
which in turn can relay signals to the gastrin-releasing cells,
histamine-releasing cells, and parietal cells. In addition, peptides and amino
acids can act directly on the gastrin-releasing enteroendocrine cells to promote
gastrin secretion. The concentration of acid in the gastric lumen is itself an
important determinant of the rate of acid secretion because H+ (acid)
directly inhibits gastrin secretion. It also stimulates the release of
somatostatin from D cells in the stomach wall. Somatostatin then acts on the
parietal cells to inhibit acid secretion; it also inhibits the release of
gastrin and histamine. The net result is a negative feedback control of acid
secretion. As the contents of the gastric lumen become more acidic, the stimuli
that promote acid secretion decrease.
Increasing the protein content of a meal increases acid secretion.
This occurs for two reasons. First, protein ingestion increases the
concentration of peptides in the lumen of the stomach. These peptides, as we
have seen, stimulate acid secretion through their actions on gastrin. The second
reason is more complicated and reflects the effects of proteins on luminal
acidity. During the cephalic phase, before food enters the stomach, the H+
concentration in the lumen increases because there are few buffers present to
bind any secreted H+. Thereafter, the rate of acid secretion soon
decreases because high acidity reflexively inhibits acid secretion. The protein
in food is an excellent buffer, however, so as it enters the stomach, the H+
concentration decreases as H+ binds to proteins and begins to
denature them. This decrease in acidity removes the inhibition of acid
secretion. The more protein in a meal, the greater the buffering of acid and the
more acid secreted. We now come to the intestinal phase that controls acid
secretion—the phase in which stimuli in the early portion of the
small intestine influence acid secretion by the stomach. High acidity in the
duodenum triggers reflexes that inhibit gastric acid secretion. This inhibition
is beneficial because the digestive activity of enzymes and bile salts in the
small intestine is strongly inhibited by acidic solutions. This reflex limit
gastric acid production when the H+
concentration in the duodenum increases due to the entry of chyme from the
stomach.
Acid, distension, hypertonic solutions, solutions containing amino acids, and
fatty acids in the small intestine reflexively inhibit gastric acid secretion.
The extent to which acid secretion is inhibited during the intestinal phase
varies, depending upon the amounts of these substances in the intestine; the net
result is the same, however—balancing the secretory activity of the stomach with
the digestive and absorptive capacities of the small intestine. The inhibition
of gastric acid secretion during the intestinal phase is mediated by short and
long neural reflexes and by hormones that inhibit acid secretion by influencing
the four signals that directly control acid secretion: ACh, gastrin, histamine,
and somatostatin. The hormones released by the intestinal tract that reflexively
inhibit gastric activity are collectively called enterogastrones and
include secretin and CCK.
Control of HCl Secretion during a Meal
|
Stimuli
|
Pathways
|
Result
|
|
Cephalic phase
|
Parasympathetic nerves to enteric nervous system |
↑ HCl secretion |
|
Sight, Smell, Taste, Chewing |
|
|
|
Gastric contents (gastric phase)
|
Long and short neural reflexes and direct stimulation |
↑ HCl secretion |
|
Distension |
of gastrin secretion |
|
|
↑ Peptides |
|
|
|
↓ H1 concentration |
|
|
|
Intestinal contents (intestinal phase)
|
Long and short neural reflexes; secretin, CCK, and other
duodenal |
↓ HCl secretion |
|
Distension |
hormones |
|
|
↑ H1 concentration |
|
|
|
↑ Osmolarity |
|
|
|
↑ Nutrient concentrations |
|
|
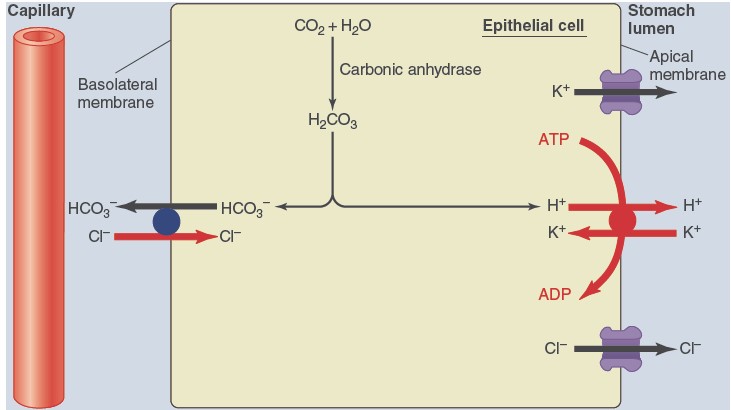
Secretion of hydrochloric acid by parietal cells. The H+ secreted
into the lumen by primary active transport is derived from H+
generated by the reaction between carbon dioxide and water, a reaction catalyzed
by the enzyme carbonic anhydrase, which is present in high concentrations in
parietal cells. The HCO3- formed by this reaction is
transported out of the parietal cell on the blood side in exchange for Cl-.
Chyme:
Semifluid mass of partially digested food expelled from stomach to intestine.
Chyle:
It is a milky body fluid containing lymph and emulsified fat or free fattyacids.
It is taken up by lymphatic vessels known as lacteals.
Enteroendocrine Cells
Enteroendocrine cells are the hormone-secreting cells in GI tract. These
are the nerve cells and glandular cells which are present in the gastric mucosa,
intestinal mucosa and the pancreatic cells.
Neuroendocrine Cells or APUD Cells
Enteroendocrine cells which secrete hormones from amines are known as amine
precursor uptake and decarboxylation cells (APUD cells) or neuroendocrine
cells. For the synthesis of GI
hormones, firs a precursor substance of an amine is taken up by these cells.
Later, this precursor substance is decarboxylated to form the amine. From this
amine, the hormone is synthesized. Because of the uptake of the amine precursor
and decarboxylation of this precursor substance, these cells are called APUD
cells. This type of cells is also present in other parts of the body,
particularly the brain, lungs and the endocrine glands.

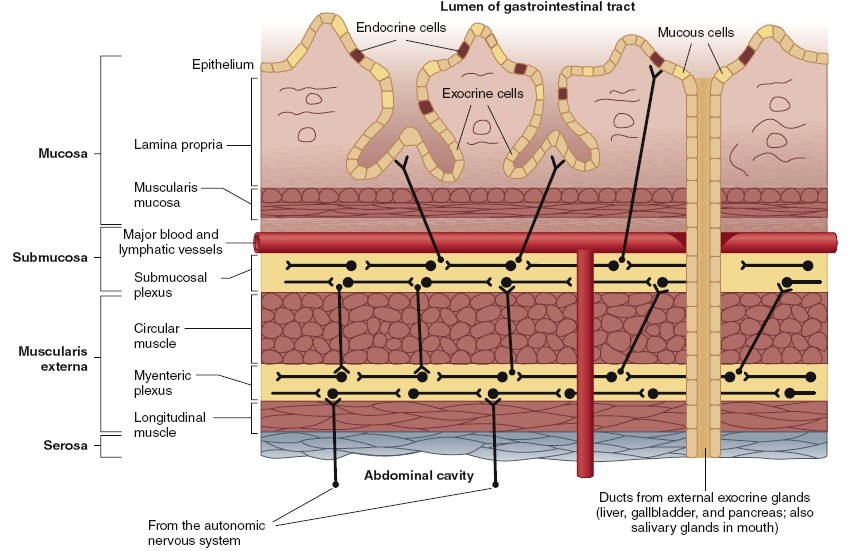
Structure of the alimentary canal in longitudinal section.
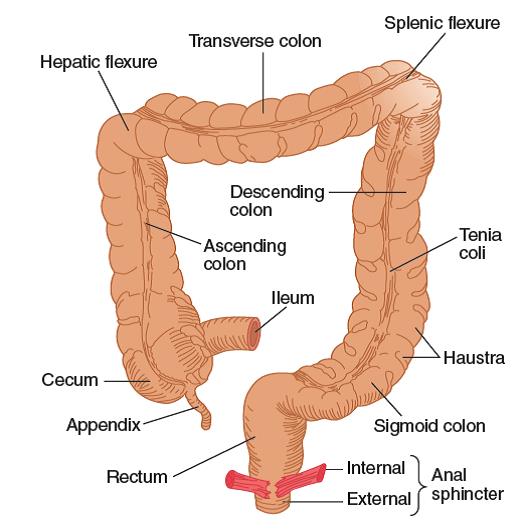
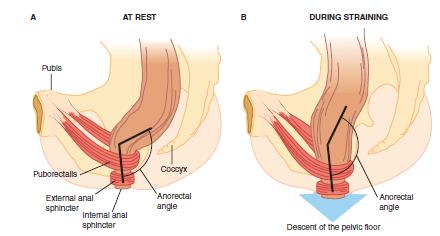
Defecation: Release of feces.
Mass movement drives the feces into sigmoid or pelvic colon. In the sigmoid
colon, the feces is stored. The desire for defecation occurs when some feces
enters rectum due to the mass movement. Usually, the desire for defecation is
elicited by an increase in the intrarectal pressure to about 20 to 25 cm H2O.
Usual stimulus for defecation is intake of liquid like coffee or tea or water.
But it differs from person to person.
Act of Defecation
Act of defecation is preceded by voluntary efforts like assuming an appropriate
posture, voluntary relaxation of external sphincter and the compression of
abdominal contents by voluntary contraction of abdominal muscles. Usually, the
rectum is empty. During the development of mass movement, the feces is pushed
into rectum and the defecation reflex is initiated. The process of defecation
involves the contraction of rectum and relaxation of internal and external anal
sphincters. Internal anal sphincter is made up of smooth muscle and it is
innervated by parasympathetic nerve fibers via pelvic nerve. External anal
sphincter is composed of skeletal muscle and it is controlled by somatic
nerve fibers, which pass through pudendal nerve. Pudendal nerve always
keeps the external sphincter constricted
and the sphincter can relax only when the pudendal nerve is inhibited.
ISOSMOTIC ABSORPTION OF WATER
Water is transported through the intestinal membrane entirely by diffusion.
Furthermore, this diffusion obeys the usual laws of osmosis. Therefore, when
the chyme is dilute enough, water is absorbed through the intestinal mucosa into
the blood of the villi almost entirely by osmosis.
Conversely, water can also be transported in the opposite direction—from
plasma into the chyme. This type of transport occurs especially when
hyperosmotic solutions are discharged from the stomach into the duodenum. Within
minutes, sufficient water usually will be transferred by osmosis to make the
chyme isosmotic with the plasma.
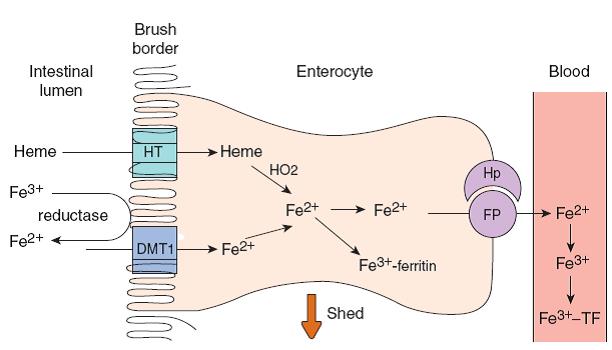
Absorption of iron.
Fe3+ is converted to Fe2+ by ferric reductase, and Fe2+
is transported into the enterocyte by the apical membrane iron transporter DMT1.
Heme is transported into the enterocyte by a separate heme transporter (HT), and
HO2 releases Fe2+ from the heme. Some of the intracellular Fe2+
is converted to Fe3+ and bound to ferritin. The rest binds to the
basolateral Fe2+ transporter ferroportin (FP) and is transported to
the interstitial fluid. The transport is aided by hephaestin (Hp). In plasma, Fe2+
is converted to Fe3+ and bound to the iron transport protein
transferrin (TF). Divalent metal transporter 1 (DMT1)
Calcium Absorption
A total of 30–80% of ingested calcium is absorbed. Through
vitamin D derivative, Ca2+ absorption is adjusted to body needs; absorption is
increased in the presence of Ca2+ deficiency and decreased in the presence of
Ca2+ excess. Ca2+ absorption is also facilitated by protein. It is inhibited by
phosphates and oxalates because these anions form insoluble salts with Ca2+ in
the intestine. Magnesium absorption is also facilitated by protein.
Vitamins Absorption
The fat-soluble vitamins A, D, E, and K are ingested as
esters and must be digested by cholesterol esterase prior to absorption. These
vitamins are also highly insoluble in the gut, and their absorption is therefore
entirely dependent on their incorporation into micelles. Most vitamins are
absorbed in the upper small intestine, but vitamin B 12 is absorbed in the
ileum. This vitamin binds to intrinsic factor, a protein secreted by the
parietal cells of the stomach, and the complex is absorbed across the ileal
mucosa. Vitamin B 12 absorption and
folate absorption are Na+ -independent, but all seven of the
remaining water-soluble vitamins—thiamin, riboflavin, niacin, pyridoxine,
pantothenate, biotin, and ascorbic acid—are absorbed by carriers that are Na+
cotransporters.
EXCRETORY SYSTEM
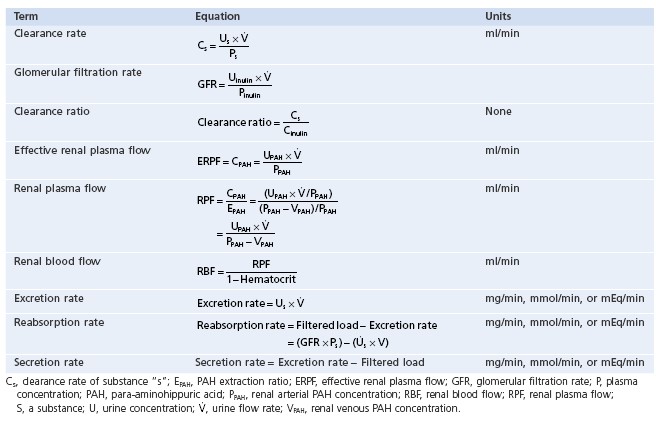
STRUCTURE OF KIDNEY
The two kidneys lie in the back of the abdominal wall but not actually in the
abdominal cavity. They are retroperitoneal, meaning they are just behind the
peritoneum, the lining of this cavity. The urine flows from the kidneys through
the ureters into the bladder and then is eliminated via the
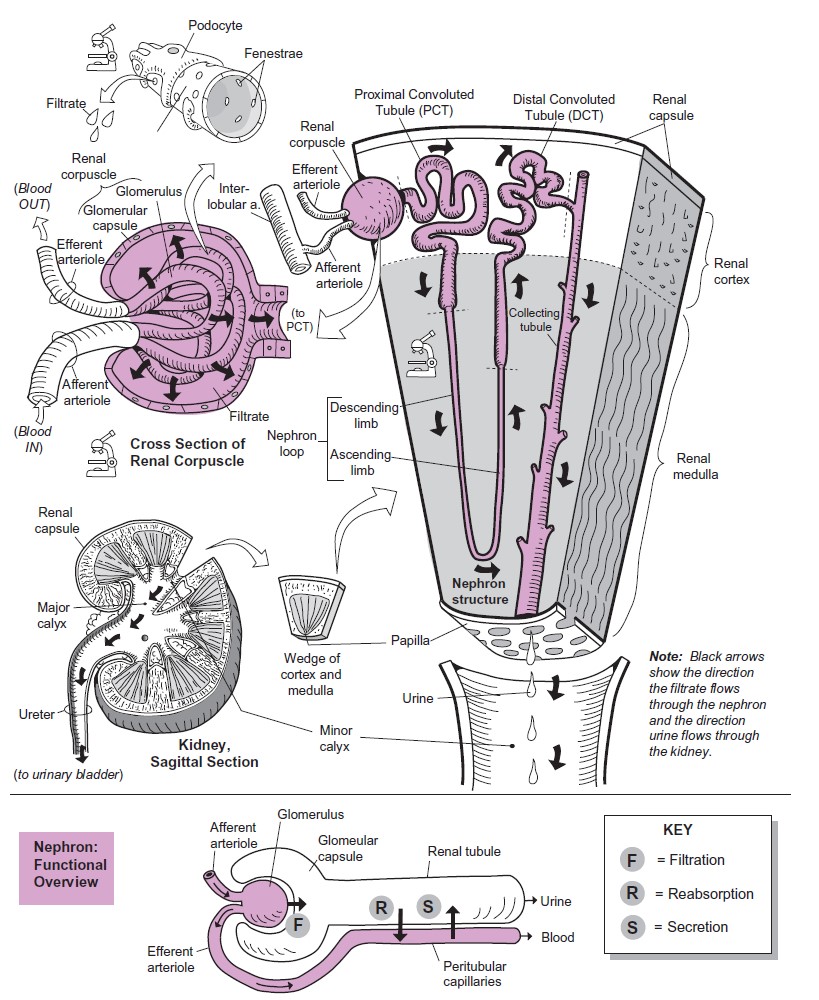
urethra. The major structural components of the kidney are shown in the
figure. The indented surface of the kidney is called the
hilum, through
which courses the blood vessels perfusing (renal artery) and draining (renal
vein) the kidneys. The nerves that
innervate the kidney and the tube that drains urine from the kidney (the ureter)
also pass through the hilum. The ureter is formed from the calyces
(singular, calyx), which are funnel-shaped structures that drain urine
into the renal pelvis, from where the urine enters the ureter. Also
notice that the kidney is surrounded by a protective capsule made of connective
tissue. The kidney is divided into an outer renal cortex and inner
renal medulla, described in more
detail later. The connection between the tip of the medulla and the calyx is
called the papilla.
Each kidney contains approximately 1 million similar functional units called
nephrons. Each nephron consists of (1) an initial filtering component called
the renal corpuscle and (2) a tubule that extends from the renal
corpuscle.
The renal tubule is a very narrow, fluid-filled cylinder made up of a single
layer of epithelial cells resting on a basement membrane. The epithelial cells
differ in structure and function along the length of the tubule, and at least
eight distinct segments are now recognized. It is customary, however, to group
two or more contiguous tubular segments when discussing function, and we will
follow this practice. The renal corpuscle forms a filtrate from blood that is
free of cells, larger polypeptides, and proteins. This filtrate then leaves the
renal corpuscle and enters the tubule. As it flows through the tubule,
substances are added to or removed from it. Ultimately, the fluid remaining at
the end of each nephron combines in the collecting ducts and exits the kidneys
as urine.
FUNCTIONS OF KIDNEYS
The kidneys perform their most important functions by filtering the plasma and
removing substances from the filtrate at variable rates, depending on the needs
of the body. Ultimately, the kidneys “clear” unwanted substances from the
filtrate (and therefore from the blood) by excreting them in the urine while
returning substances that are needed back to the blood.
Although this chapter and the next few chapters focus mainly on the control of
renal excretion of water, electrolytes, and metabolic waste products, the
kidneys serve many important homeostatic functions, including the following:
• Excretion of metabolic waste products and foreign chemicals
• Regulation of water and electrolyte balances
• Regulation of body fluid osmolality and electrolyte concentrations
• Regulation of arterial pressure
• Regulation of acid-base balance
• Regulation of erythrocyte production
• Secretion, metabolism, and excretion of hormones
• Gluconeogenesis
Excretion of Metabolic Waste Products, Foreign Chemicals, Drugs, and Hormone
Metabolites.
The kidneys are the primary means for eliminating waste products of metabolism
that are no longer needed by the body. These products include urea (from
the metabolis of amino acids), creatinine (from
muscle creatine), uric acid (from nucleic acids), end products of
hemoglobin breakdown (such as bilirubin), and metabolites of various
hormones. These waste products must be eliminated from the body as rapidly
as they are produced. The kidneys also eliminate most toxins and other foreign
substances that are either produced by the body or ingested, such as pesticides,
drugs, and food additives.
Regulation of Water and Electrolyte Balances.
For
maintenance of homeostasis, excretion of water and electrolytes must precisely
match intake. If intake exceeds excretion, the amount of that substance in the
body will increase. If intake is less than excretion, the amount of that
substance in the body will decrease. Although temporary (or cyclic) imbalances
of water and electrolytes may occur in various physiological and
pathophysiological conditions associated with altered intake or renal excretion,
the maintenance of life depends on restoration of water and electrolyte balance.
Intake of water and many electrolytes is governed mainly by a person’s eating
and drinking habits, requiring the kidneys to adjust their excretion rates to
match the intakes of various substances. The figure
shows the response of the kidneys to a sudden 10-fold increase in sodium intake
from a low level of 30 mEq/day to a high level of 300 mEq/day. Within 2 to 3
days after raising the sodium intake, renal excretion also increases to about
300 mEq/day so that a balance between intake and output is rapidly
re-established. However, during the 2 to 3 days of renal adaptation to the high
sodium intake, there is a modest accumulation of sodium that raises
extracellular fluid volume slightly and triggers hormonal changes and other
compensatory responses that signal the kidneys to increase their sodium
excretion. The capacity of the kidneys to alter sodium excretion in response to
changes in sodium intake is enormous. Experimental studies have shown that in
many people, sodium intake can be increased to 1500 mEq/day (more than 10 times
normal) or decreased to 10 mEq/day (less than one-tenth normal) with relatively
small changes in extracellular fluid volume or plasma sodium concentration. This
phenomenon is also true for water and for most
other electrolytes, such as chloride, potassium, calcium, hydrogen, magnesium,
and phosphate ions. In the next few chapters, we discuss the specific mechanisms
that permit the kidneys to perform these amazing feats of homeostasis.
Regulation of Arterial Pressure.
The kidneys play a dominant role
in long-term regulation of arterial pressure by excreting variable amounts of
sodium and water. The kidneys also contribute to short-term arterial pressure
regulation by secreting hormones and vasoactive factors or substances (e.g.,
renin) that lead to the formation of vasoactive products (e.g., angiotensin
II).
Regulation of Acid-Base Balance.
The kidneys contribute to acid-base regulation, along with the lungs and body
fluid buffers, by excreting acids and by regulating the body fluid buffer
stores. The kidneys are the only means of eliminating from the body certain
types of acids, such as sulfuric acid and phosphoric acid, generated by the
metabolism of proteins.
Regulation of Erythrocyte Production.
The kidneys secrete
erythropoietin, which stimulates the production of red blood cells by
hematopoietic stem cells in the bone marrow. One important stimulus for
erythropoietin secretion by the kidneys is hypoxia. The kidneys normally
account for almost all the erythropoietin secreted into the circulation. In
people with severe kidney disease or who have had their kidneys removed and have
been placed on hemodialysis, severe anemia develops as a result of decreased
erythropoietin production.
Regulation of 1,25-Dihydroxyvitamin D3 Production.
The kidneys produce the active form of vitamin D, 1,25-dihydroxyvitamin D3
(calcitriol), by hydroxylating this vitamin at the “number 1” position.
Calcitriol is essential for normal calcium deposition in bone and calcium
reabsorption by the gastrointestinal tract. Calcitriol plays an important role
in calcium and phosphate regulation.
Glucose Synthesis.
The kidneys synthesize glucose from amino acids and other precursors during
prolonged fasting, a process referred to as gluconeogenesis. The kidneys’
capacity to add glucose to the blood during prolonged periods of fasting rivals
that of the liver. With chronic kidney disease or acute failure of the kidneys,
these homeostatic functions are disrupted and severe abnormalities of body fluid
volumes and composition rapidly occur. With complete renal failure, enough
potassium, acids, fluid, and other substances accumulate in the body to cause
death within a few days, unless clinical interventions such as hemodialysis are
initiated to restore, at least partially, the body fluid and electrolyte
balances.
STRUCTURE OF NEPHRON
The nephron is the functional unit of the kidney responsible for the
formation of urine. Each kidney contains more than a million nephrons. A nephron
consists of small tubes, or tubules, and associated small blood vessels.
Fluid formed by capillary filtration enters the tubules and is subsequently
modified by transport processes; the resulting fluid that leaves the tubules is
urine.
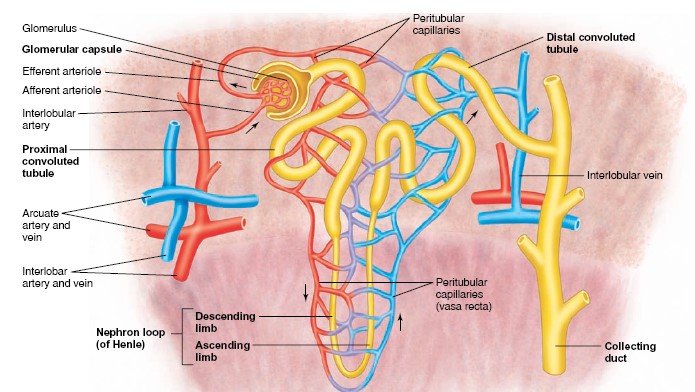
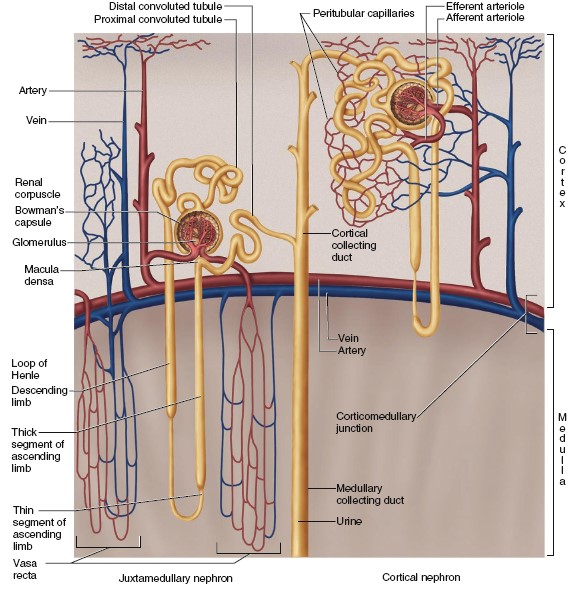
Renal Blood Vessels
Arterial blood enters the kidney through the renal artery, which divides
into interlobar arteries that pass between the pyramids through the renal
columns. Arcuate arteries branch from the interlobar arteries at the
boundary of the cortex and medulla. A number of interlobular arteries
radiate from the arcuate arteries into the cortex and subdivide into numerous
afferent arterioles, which are microscopic. The afferent arterioles deliver
blood into glomeruli — capillary networks that produce a blood filtrate
that enters the urinary tubules. The blood remaining in a glomerulus leaves
through an efferent arteriole, which delivers the blood into another
capillary network—the peritubular capillaries surrounding the renal
tubules.
This arrangement of blood vessels is unique. It is the only one in the body in
which a capillary bed (the glomerulus) is drained by an arteriole rather than by
a venule and delivered
to a second capillary bed located downstream (the peritubular capillaries).
Blood from the peritubular capillaries is drained into veins that parallel the
course of the arteries in the kidney. These veins are called the interlobular
veins, arcuate veins, and interlobar veins. The interlobar veins
descend between the pyramids, converge, and leave the kidney as a single
renal vein, which empties into the inferior vena cava.
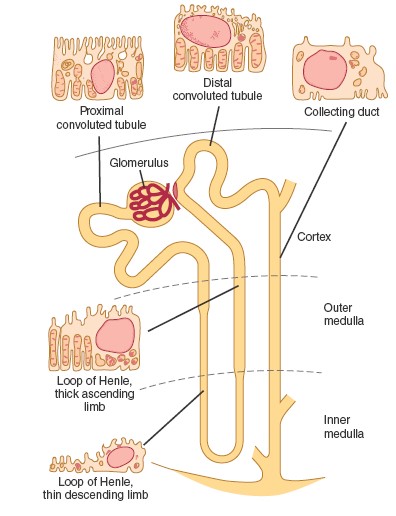
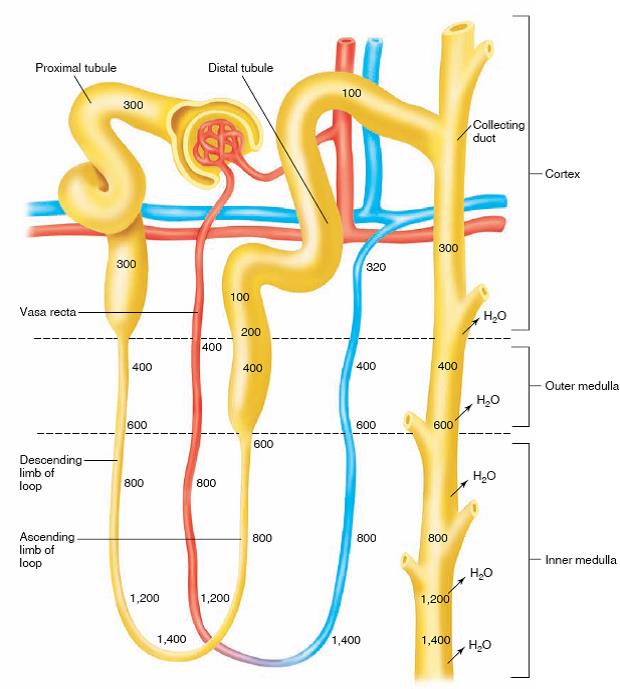
Nephron Tubules
The tubular portion of a nephron consists of a glomerular capsule, a
proximal convoluted tubule, a descending limb of the loop of Henle,
an ascending limb of the loop of Henle, and a distal convoluted tubule.
The glomerular (Bowman’s) capsule surrounds the glomerulus. The
glomerular capsule and its associated glomerulus are located in the cortex of
the kidney and together constitute the renal corpuscle. The glomerular
capsule contains an inner visceral layer of epithelium around the glomerular
capillaries and an outer parietal layer. The space between these two layers is
continuous with the lumen of the tubule and receives the glomerular filtrate, as
will be described in the next section.
Filtrate that enters the glomerular capsule passes into the lumen of the
proximal convoluted tubule. The wall of the proximal convoluted tubule
consists of a single layer of cuboidal cells containing millions of microvilli;
these microvilli increase the surface area for reabsorption. In the process of
reabsorption, salt, water, and other molecules needed by the body are
transported from the lumen, through the tubular cells and into the surrounding
peritubular capillaries.
The glomerulus, glomerular capsule, and convoluted tubule are located in the
renal cortex. Fluid passes from the proximal convoluted tubule to the nephron
loop, or loop of Henle. This fluid is carried into the medulla in the
descending limb of the loop and returns to the cortex in the ascending
limb of the loop. Back in the cortex, the tubule again becomes coiled and is
called the distal convoluted tubule.
The distal convoluted tubule is shorter than the proximal tubule and has
relatively few microvilli. The distal convoluted tubule terminates as it empties
into a collecting duct. The two principal types of nephrons are classified
according to their position in the kidney and the lengths of their loops of
Henle. Nephrons that originate in the inner one-third of the cortex—called
juxtamedullary nephrons because they are next to the medulla—have longer
loops of Henle than the more numerous cortical nephrons, which originate
in the outer two-thirds of the cortex. The juxtamedullary nephrons play an
important role in the ability of the kidney to produce a concentrated urine. A
collecting duct receives fluid from the distal convoluted tubules of
several nephrons. Fluid is then drained by the collecting duct from the cortex
to the medulla as the collecting duct passes through a renal pyramid. This
fluid, now called urine, passes into a minor calyx. Urine is then funneled
through the renal pelvis and out of the kidney in the ureter.
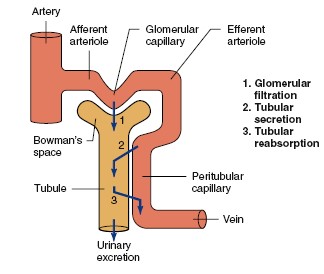
URINE FORMATION
Urine formation begins with the filtration of plasma from the glomerular
capillaries into Bowman’s space. This process is termed glomerular
filtration, and the filtrate is called the glomerular filtrate. It is
cell-free and, except for larger proteins, contains all the substances in
virtually the same concentrations as in plasma. This type of filtrate, in which
only low-molecular weight solutes appear, is also called an ultrafiltrate.
During its passage through the tubules, the filtrate’s composition is
altered by movements of substances from the tubules to the peritubular
capillaries, and vice versa. When the direction of movement is from tubular
lumen to peritubular capillary plasma, the process is called tubular
reabsorption or, simply, reabsorption. Movement in the opposite
direction—that is, from peritubular plasma to tubular lumen—is called tubular
secretion or, simply, secretion. Tubular secretion is also used to denote
the movement of a solute from the cell interior to the lumen in the cases in
which the kidney tubular cells themselves generate the substance.
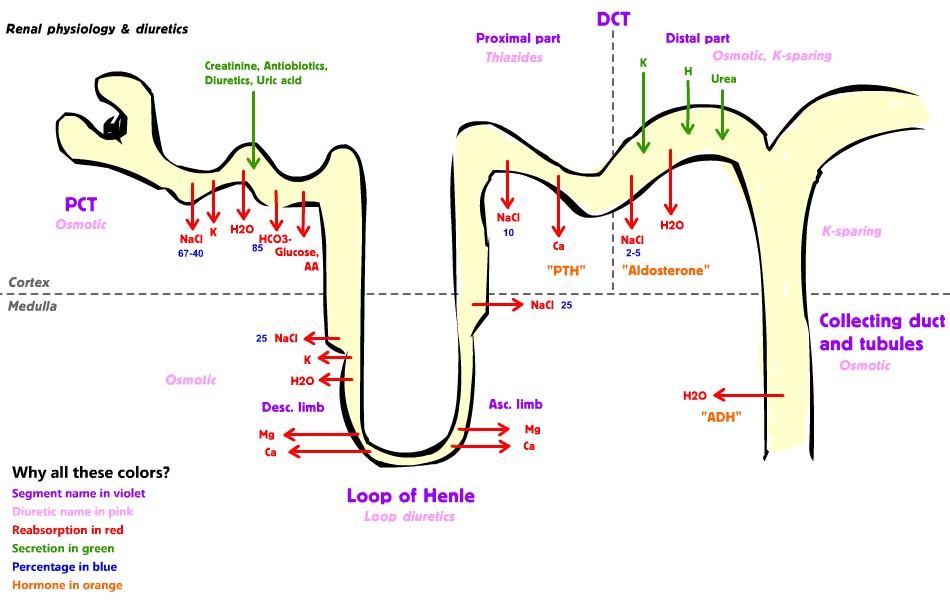
In a day 180L/day filtrate formed but 99% of them were reabsorbed only 1
– 2 L/day is formed as urine.
To summarize, a substance can gain entry to the tubule and be excreted in the
urine by glomerular filtration or tubular secretion or both. Once in the tubule,
however, the substance does not have to be excreted but can be partially or
completely reabsorbed. Thus, the amount of any substance excreted in the urine
is equal to the amount filtered plus the amount secreted minus the amount
reabsorbed.
Urine flow through the ureters to the bladder is propelled by contractions of
the ureter wall smooth muscle. The urine is stored in the bladder and
intermittently ejected during urination, or micturition.
Incontinence
is the involuntary release of urine, which can be a disturbing
problem both socially and hygienically. The most common types are stress
incontinence (due to sneezing, coughing, or exercise) and urge
incontinence (associated with the desire to urinate). Incontinence is
more common in women and may occur one to two times per week in more than 25% of
women older than 60. It is very common in older women in nursing homes and
assisted-living facilities
Glomerular filtration
Urine formation begins when a large amount of fluid that is virtually free of
protein is filtered from the glomerular capillaries into Bowman’s capsule. Most
substances in the plasma, except for proteins, are freely filtered, so their
concentration in the glomerular filtrate in Bowman’s capsule is almost the same
as in the plasma.
As stated previously, the glomerular filtrate—that
is, the fluid in Bowman’s space—normally contains no cells but contains all
plasma substances except proteins in virtually the same concentrations as in
plasma. This is because glomerular filtration is a bulk-flow process in which
water and all low-molecular-weight substances (including smaller polypeptides)
move together. Most plasma proteins—the albumins and globulins—are excluded from
the filtrate in a healthy kidney. One reason for their exclusion is that the
renal corpuscles restrict the movement of such highmolecular-weight substances.
A second reason is that the filtration pathways in the corpuscular membranes are
negatively charged, so they oppose the movement of these plasma proteins, most
of which are also negatively charged.
The only exceptions to the generalization that all nonprotein plasma substances
have the same concentrations in the glomerular filtrate as in the plasma are
certain low-molecular-weight substances that would otherwise be filterable but
are bound to plasma proteins and therefore not filtered. For example, the half
of the plasma calcium bound to plasma proteins and virtually all of the plasma
fatty acids that are bound to plasma protein are not filtered.
The volume of fluid filtered from the glomeruli into Bowman’s space per unit
time is known as the glomerular filtration rate (GFR). Starling
forces are (1) the hydrostatic pressure difference across the capillary wall
that favors filtration and (2) the protein concentration difference across the
wall that creates an osmotic force that opposes filtration.
The range of GFR is 90 – 140 ml/min for men and 80 – 125 ml/min for
women. The range with respect to day
is 180L/day for men and 170L/day for women.
Tubular Reabsorption
Recall that a major function of the kidneys is to eliminate soluble waste
products. To do this, the blood is filtered in the glomeruli. One consequence of
this is that substances necessary for normal body functions are filtered from
the plasma into the tubular fluid. To prevent the loss of these important
nonwasted products, the kidneys have powerful mechanisms to reclaim useful
substances from tubular fluid while simultaneously allowing
waste products to be excreted. The reabsorptive rates for water and many ions,
although also very high, are under physiological control. For example, if water
intake is decreased, the kidneys can increase water reabsorption to minimize
water loss. In contrast to glomerular filtration, the crucial steps in tubular
reabsorption—those that achieve movement of a substance from tubular lumen to
interstitial fluid—do not occur by bulk flow because there are inadequate
pressure differences across the tubule and limited permeability of the tubular
membranes. Instead, two other processes are involved. (1) The reabsorption of
some substances from the tubular lumen is by diffusion, often across the tight
junctions connecting the tubular epithelial cells.
(2) The reabsorption of all other substances involves mediated transport, which
requires the participation of transport proteins in the plasma membranes of
tubular cells. The final step in reabsorption is the movement of substances from
the interstitial fluid into peritubular capillaries that occurs by a combination
of diffusion and bulk flow.
Reabsorption by Diffusion
The reabsorption of urea by the proximal tubule provides an example of passive
reabsorption by diffusion. An analysis of urea concentrations in the proximal
tubule will help clarify the mechanism. As stated earlier, urea is a waste
product; however, as you will learn shortly, some urea is reabsorbed from the
proximal tubule in a process that facilitates water reabsorption farther down
the nephron. Because the corpuscular
membranes are freely filterable to urea, the urea concentration in the fluid
within Bowman’s space is the same as that in the peritubular capillary plasma
and the interstitial fluid surrounding the tubule. Then, as the filtered fluid
flows through the proximal tubule, water reabsorption occurs (by mechanisms to
be described later). This removal of water increases the concentration of urea
in the tubular fluid so it is higher than in the interstitial fluid and
peritubular capillaries. Therefore, urea diffuses down this concentration
gradient from tubular lumen to peritubular capillary. Urea reabsorption is thus
dependent upon the reabsorption of water.
Reabsorption by Mediated Transport
Many solutes are reabsorbed by primary or secondary active transport. These
substances must first cross the apical membrane (also called the
luminal membrane) that separates the tubular lumen from the cell interior.
Then, the substance diffuses through the cytosol of the cell and, finally,
crosses the basolateral membrane, which begins at the tight junctions and
constitutes the plasma membrane of the sides and base of the cell. The movement
by this route is termed transcellular epithelial transport. A substance
does not need to be actively transported across both the apical and
basolateral membranes in order to be actively transported across the overall
epithelium, moving from lumen to interstitial fluid against its electrochemical
gradient. For example, Na+ moves “downhill” (passively) into the cell
across the apical membrane through specific channels or transporters and then is
actively transported “uphill” out of the cell across the basolateral membrane
via Na+/K+-ATPases in this membrane. The reabsorption of
many substances is coupled to the reabsorption of Na+. The
cotransported substance moves uphill into the cell via a secondary active
cotransporter as Na+ moves downhill into the cell via this same
cotransporter. This is precisely how glucose, many amino acids, and other
organic substances undergo tubular reabsorption. The reabsorption of several
inorganic ions is also coupled in a variety of ways to the reabsorption of Na+.
Many of the mediated-transport-reabsorptive systems in the renal tubule have a
limit to the amounts of material they can transport per unit time known as the
transport maximum (Tm). This is because the binding sites
on the membrane transport proteins become saturated when the concentration of
the transported substance increases to a certain level. An important example is
the secondary active-transport proteins for glucose, located in the proximal
tubule.
The pattern described for glucose is also true for a large number of other
organic nutrients. For example, most amino acids and water-soluble vitamins are
filtered in large amounts each day, but almost all of these filtered molecules
are reabsorbed by the proximal tubule. If the plasma concentration becomes high
enough, however, reabsorption of the filtered load will not be as complete and
the substance will appear in larger amounts in the urine. Thus, people who
ingest very large quantities of vitamin C have increased plasma concentrations
of vitamin C. Eventually, the filtered load may exceed the tubular reabsorptive
Tm for this substance, and any additional ingested vitamin C is excreted
in the urine.
Tubular Secretion
Tubular secretion moves substances from peritubular capillaries into the tubular
lumen. Like glomerular filtration, it constitutes a pathway from the blood into
the tubule. Like reabsorption, secretion can occur by diffusion or by
transcellular mediated transport. The most important substances secreted by the
tubules are H+
and K+. However, a large number of normally occurring organic anions,
such as choline and creatinine, are also secreted; so are many foreign chemicals
such as penicillin. Active secretion of a substance requires active transport
either from the blood side (the interstitial fluid) into the tubule cell (across
the basolateral membrane) or out of the cell into the lumen (across the apical
membrane). As in reabsorption, tubular secretion is usually coupled to the
reabsorption of Na+.
Secretion from the interstitial space into the tubular fluid, which draws
substances from the peritubular capillaries, is a mechanism to increase the
ability of the kidneys to dispose of substances at a higher rate rather than
depending only on the filtered load.
Metabolism by the Tubules
We noted earlier that, during fasting, the cells of the renal tubules synthesize
glucose and add it to the blood. They can also catabolize certain organic
substances, such as peptides, taken up from either the tubular lumen or
peritubular capillaries. Catabolism eliminates these substances from the body
just as if they had been excreted into the urine.
Counter
Current Mechanism
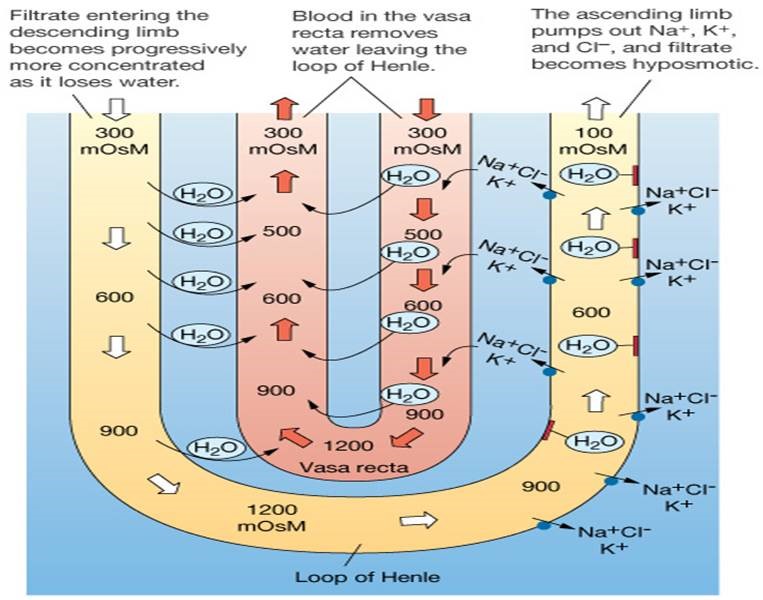
Counter current system:
System in renal medulla that facilitates concentration of urine as it passes
through renal tubules. Counter
current multiplier is an active process responsible for osmotic gradient to
produce concentrated urine. It is otherwise called as positive feedback
mechanism. Counter current exchanger refers to the exchange of material between
two flowing bodies.
The following progression of steps will occur:
1.
The interstitial fluid is now a little hypertonic due to the NaCl pumped out of
the ascending limb.
2.
Because of the slightly hypertonic interstitial fluid, some water leaves the
descending limb by osmosis (and enters the blood) as the filtrate goes deeper
into the medulla. This makes the filtrate somewhat hypertonic when it reaches
the ascending limb.
3.
The now higher NaCl concentration of the filtrate that enters the ascending limb
allows it to pump out more NaCl than it did before, because more NaCl is now
available to the carriers. The interstitial fluid now becomes yet more
concentrated.
4.
Because the interstitial fluid is more concentrated than it was in step 2, more
water is drawn out of the descending limb by osmosis, making the filtrate even
more hypertonic when it reaches the ascending limb.
5.
Step 3 is repeated, but to a greater extent because of the higher NaCl
concentration delivered to the ascending limb.
6.
This progression continues until the maximum concentration is reached in the
inner medulla. This maximum is determined by the capacity of the active
transport pumps working along the lengths of the thick segments of the ascending
limbs.