COMPLEMENTS
Complements play a vital role in humoral and innate immunity. Complements defined as the components that provide activity of blood serum that completes the action of antibody.
The name of the complement system is derived from experiments performed by Jules Bordet shortly after the discovery of antibodies. He demonstrated that if fresh serum containing an antibacterial antibody was added to the bacteria at physiologic temperature, the bacteria were lysed. If, however, the serum was heated to 56*C or more, it lost its lytic capacity. This loss of lytic capacity was not due to decay of antibody activity because antibodies are heat stable and even heated serum was capable of agglutinating the bacteria. Bordet concluded that the serum must contain another heat labile component that assists or complements the lytic function of antibodies and this component was later given the name complement.
The proteins and glycoproteins that compose the complement system are synthesized mainly by liver hepatocytes, although significant amounts are also produced by blood monocytes, tissue macrophages, and epithelial cells of the gastrointestinal and genitourinary tracts. These components constitute 5% (by weight) of the serum globulin fraction. Most circulate in the serum in functionally inactive forms as proenzymes, or zymogens, which are inactive until proteolytic cleavage, which removes an inhibitory fragment and exposes the active site. The complement-reaction sequence starts with an enzyme cascade. Complement components are designated by numerals (C1–C9), by letter symbols (e.g., factor D), or by trivial names (e.g., homologous restriction factor). Peptide fragments formed by activation of a component are denoted by small letters. In most cases, the smaller fragment resulting from cleavage of a component is designated “a” and the larger fragment designated “b” e.g., C3a, C3b; with the exception of C2 where C2a is the larger cleavage fragment. The larger fragments bind to the target near the site of activation, and the smaller fragments diffuse from the site and can initiate localized inflammatory responses by binding to specific receptors. The complement fragments interact with one another to form functional complexes. Those complexes that have enzymatic activity are designated by a bar over the number or symbol e.g., C4b2a, C3bBb.
COMPLEMENT PATHWAY:
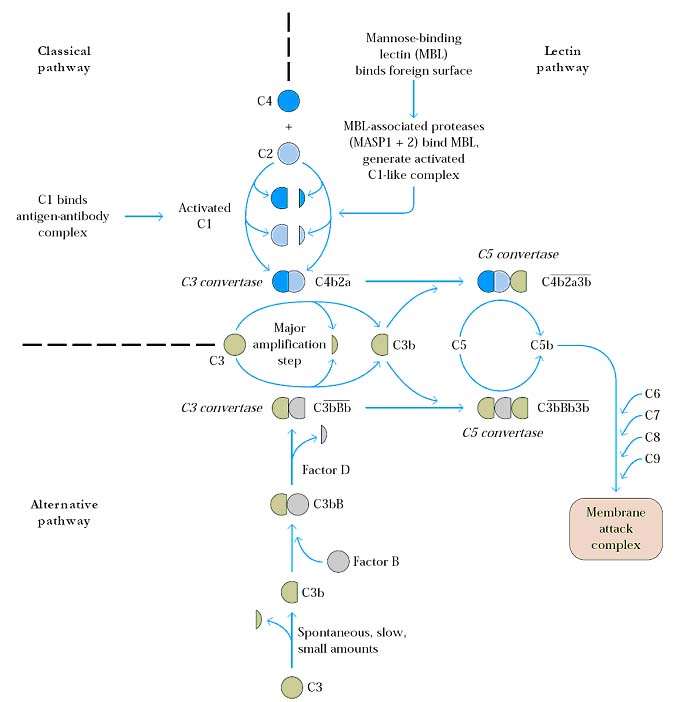
There are three major pathways of complement activation namely classical pathway which is activated by certain isotypes of antibodies bound to antigens; the alternative pathway which is activated on microbial cell surface in the absence of antibody and the lectin pathway which is activated by a plasma lectin that binds to mannose residues on microbes. The alternative and lectin pathways are effector mechanisms of innate immunity whereas the classical pathway is a mechanism of humoral immunity.

CLASSICAL PATHWAY:
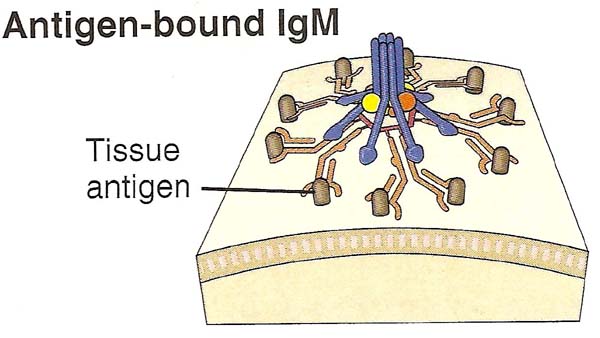
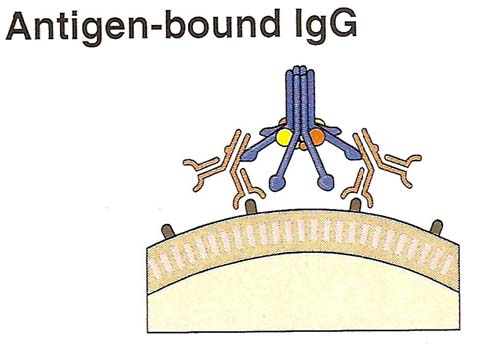
The classical pathway is initiated by binding of complement protein C1 to the CH2 domains of Ig G or the CH3 domains of Ig M molecules that have bound antigen. C1 is a large, multimeric protein complex composed of C1q, C1r and C1s subunits; C1q binds to the antibody and C1r and C1s are proteases which are tetrameric in nature.
Binding of two or more of the globular heads of C1q to the Fc regions of Ig G or Ig M leads to enzymatic activation of he associated C1r; which cleaves and activates C1s. Activated C1s cleaves the next protein in the cascade, C4 to generate C4a and C4b. C4b has an internal thioester bond that forms covalent amide or ester linkages with the antigen-antibody complex or with the adjacent surface of a cell to which the antibody bound. This attachment of C4b ensures that classical pathway activation proceeds on a cell surface or immune complex. C2 binds to C4b where the C2 is then cleaved by the neighboring C1s; the smaller fragment C2b diffuses away. The resulting C4b2a, C3 convertase formed.
The native C3 complement consists of two polypeptide chains a and b. Hydrolysis of a short fragment (C3a) from the amino terminus of the a-chain by the C3 convertase generates C3b. A single C3 convertase molecule can generate over 200 molecules of C3b. Some of the C3b binds to C4b2a, to form a trimolecular complex C4b2a3b called C5 convertase. It initiates the MAC (Membrane Attack Complex) formation.
ALTERNATIVE PATHWAY (PROPERDIN PATHWAY):
The alternative pathway of complement activation results in the proteolysis of C3 and the stable attachment of its breakdown product of C3b to microbial surfaces, without a role of antibody. C3 cleavage takes place at slower rate. After C3b formation, it may bind to either cell surface or microbial surface. Some C3b present in unbound state (Fluid phase) which is inactive and complement activation stops. The bound next binds a plasma protein called Factor B and after it is bound, Factor B is cleaved by a plasma serine protease called Factor D to generate a fragment called Bb that remains attached to C3b while Ba released. The C3bBb complex is the alternative pathway C3 convertase and it functions to cleave more C3 molecules. The C3bBb complex is stabilized by Properdin. Without Properdin, this complex was stable only for 5 minutes whereas with Properdin this complex stable for 30 minutes. The attachment is favored while C3bBb complex present on microbial cells but opposed to normal host cells. Some of the C3b molecules generated by the alternative pathway C3 convertase bind to C3 and forms C3bBb3b complex and C3a. The new complex C3bBb3b functions as the alternative pathway C5 convertase to cleave C5 and initiate the MAC formation.
LECTIN PATHWAY (MANNAN BINDING LECTIN (MBL) PATHWAY):
Lectins are proteins that recognize and bind to specific carbohydrate targets ex; MBL targets mannose. The lectin pathway is activated by the binding of mannose binding Lectin (MBL) to mannose residues on glycoproteins or carbohydrates on the surface of microbes ex: Salmonella, Candida albicans and Neiseeria Strains. MBL is an acute phase protein produced in inflammatory responses. Its function in complement pathway is similar to that of C1q which it resembles in structure. After MBL binds to the surface of a cell or pathogen, MBL-associated serine proteases, MASP-1 and MASP-2, bind to MBL. The active complex formed by this association causes cleavage and activation of C4 and C2. Remaining reactions are similar to classical pathway. The MASP-1 and MASP-2 proteins have structural similarity to C1r and C1s and mimic their activities. Lectin pathway does not depend on antibody for its activation like alternative pathway.
MAC FORMATION (COMMON PATHWAY):
C5 convertase generated by the classical, alternative and lectin pathway initiate activation of late components of complement system, C5, C6, C7, C8 and C9, which involved in the formation of the cytocidal membrane attack complex. The C5 convertase converts C5 into C5b and C5a. C5a released whereas C5b binds to surface of the target cell and it is extremely labile and become inactive within 2 minutes unless C6 binds to it and stabilizes its activity. C5b binds to C6 to form C5b6 complex, it is in fluid phase. As C5b6 binds to C7, the resulting complex C5b67 undergoes a hydrophilic-amphiphilic structural transition that exposes regions which provide binding sites for membrane phospholipids. So, it insert into the lipid bilayer of membrane. The C8 protein is a trimer composed of three chains, one of which binds to the C5b67 complex. This activates C8 like C7 and it inserts into lipid bilayer. The C5b678 complex creates a small pore 10A* in diameter, formation of this pore can lead to lysis of RBC but not of nucleated cell.
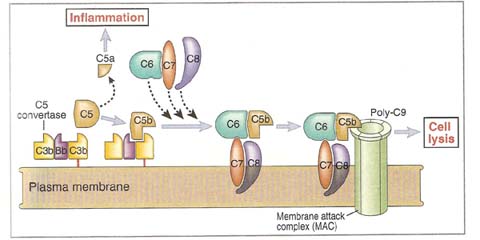
The final step in formation of the MAC is the binding and polymerization of C9 (10 – 15 molecules), a perforin-like molecule to C5b678 complex. During polymerization, the C9 molecules undergo a hydrophilic-amphiphilic transition, so that they too can insert into the membrane. The completed MAC produces pore of about 70 – 100A* size. Since ions like Ca2+ and small molecules can diffuse freely through the central channel of the MAC, the cell cannot maintain its osmotic stability and is killed by an influx of water and loss of electrolytes.
FUNCTION OF COMPLEMENTS:
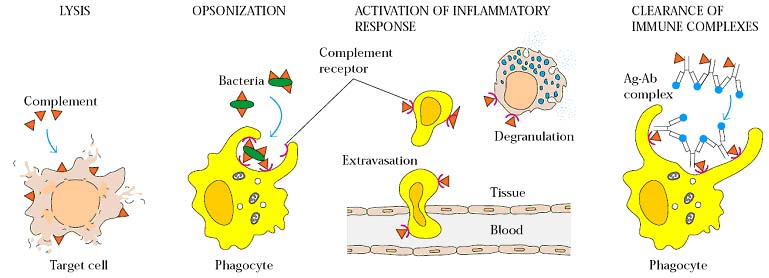
The biological activities of complement affect both innate and acquired immunity and reach for beyond the original observations of antibody mediated lysis of bacteria and RBCs. After activation, components of complement system carry out a number of basic functions including:
i) Cell lysis by MAC
ii) Opsonization and phagocytosis
iii) Activation of Inflammatory Response
iv) Viral Neutralization
v) Removal of Immune complex
Bovine serum contains an unusual protein called conglutinin (K) which causes clumping of particles or cells coated with complements, a process known as conglutination. Conglutinin reacts exclusively with bound C3. Though conglutinin behaves as an antibody to C3, it is not an immunoglobulin and requires Ca2+ for its activity. Antibodies with conglutinin-like activity (immunoconglutinin IK) can be produced by immunization with complement coated materials. High IK levels have been noticed in the saliva and jejunal secretions.
i) Cell lysis by MAC:
The MAC formed by complement activation can lyse gram negative bacteria, parasites, viruses, erythrocytes and nucleated cells.
ii) Opsonization and phagocytosis:
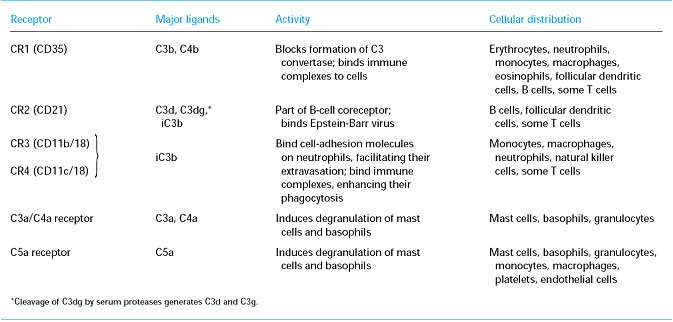
C3b is the major opsonin of the complement system, although C4b and iC3b also have opsonizing activity. The amplification that occurs with C3 activation results in a coating of C3b on immune complexes and particulate antigens. Phagocytic cells as well as some other cells, express complement receptors that bind C3b, C4b or iC3b. Antigen coated with C3b binds to cells bearing CR1. If the cells are a phagocyte, phagocytosis occurs. C5a increases the expression of CR1 on the surface of phagocytes. Recent studies indicate that complement fragment C3b act as an adjuvant when coupled with protein antigens because C3b targets the antigen directly to the phagocyte, enhancing the initiation of antigen processing and accelerating specific antibody production.
iii) Activation of Inflammatory Response:
The proteolytic complement fragments C5a, C4a and C3a induce acute inflammation by activating mast cells and neutrophils. All three peptides bind to mast cells and induce Degranulation, with the release of vasoactive mediators such as histamine. These peptides are also called anaphylatoxins because the mast cell reactions. They trigger characteristics of anaphylaxis.
In neutrophils, C5a stimulates motility, firm adhesion to endothelial cells and at high doses, stimulation of the respirator burst and production of reactive oxygen intermediates (ROI). The activities of these highly reactive anaphylactins are regulated by a serum protease called carboxypeptidase – N, which cleaves an Arg residues from the C-terminus of the molecules, yielding so called des-Arg forms. The des-Arg forms of C3a and C4a are completely inactive while that of C5a retains about 10% of its chemotactic activity and 1% of its ability to cause smooth muscle contraction.
iv) Viral Neutralization:
The complement system mediates viral neutralization by many mechanisms. First one is viral neutralization by the formation of larger viral aggregates due to the attachment of complements on its surface. Viral aggregates reduce the number of infectious viral particles. Ex: polyoma virus coated with antibody is neutralized when serum containing activated C3 is added.
Neutralization also achieved by forming thick protein on viral particles by complements and antibodies. Third mechanism is activation of phagocytosis of virus. The deposits of antibodies and complement on viral particle facilitate binding of the viral particle to cell processing Fc or type 1 complement receptor (CR1). If the cell with receptor is phagocyte then phagocytosis occurs. Finally, complement is effective4 in lysing most, if not all, enveloped viruses, resulting in fragmentation of envelop and disintegration of nucleocapsid.
v) Removal of Immune Complex:
After opsonization of microbes by complements mainly by C3b, they are recognized by erythrocytes through CR1 on its surface in circulation. When these immune complex bound erythrocytes reach liver and spleen, immune complex is received from erythrocytes by macrophages through complement receptors. Then these immune complexes removed by phagocytosis. Thus complements plays vital role in the removal of immune complexes which plays vital role in certain autoimmune disorders like SLE and type – III hypersensitivity.
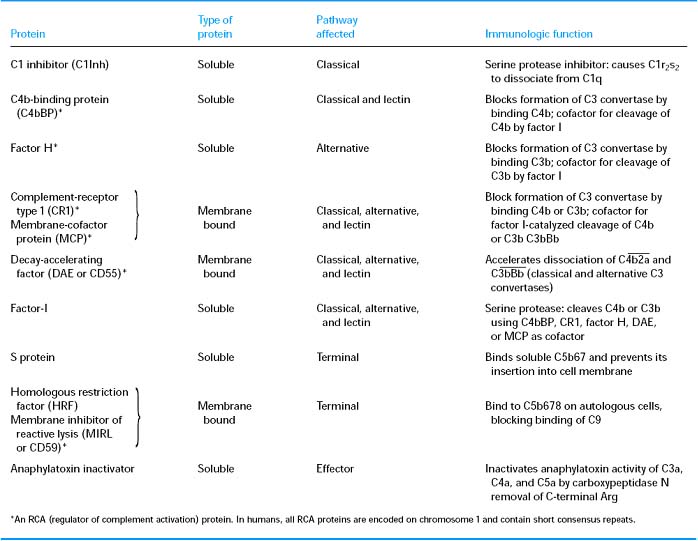
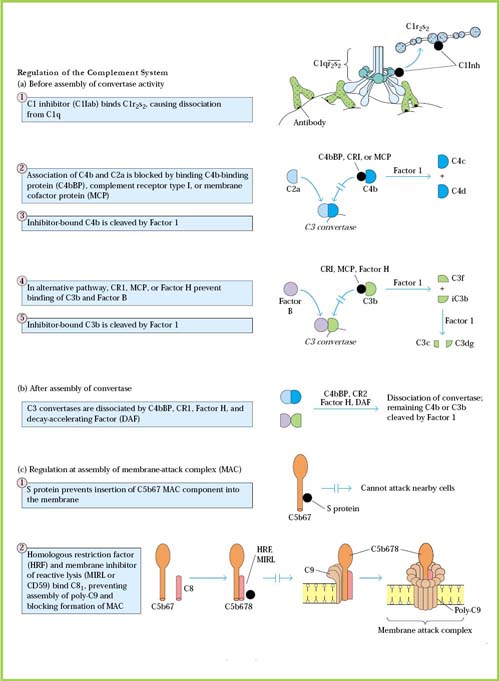
COMPLEMENT REGULATORS:
COMPLEMENT RECEPTORS:
MICROBIAL EVASION:
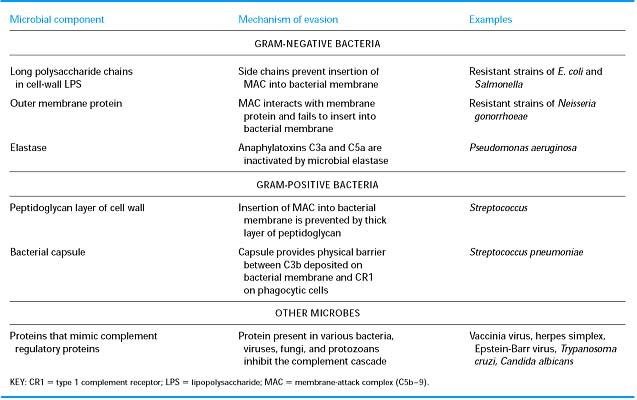
Innocent Bystander Lysis:
When C5b67 complex produced during complement activation pathways, they bind to surface of membrane of nearby cells and results in lysis. If they bound to normal cells and lyse the cells then the phenomenon is known as “Innocent Bystander Lysis”. But in nature, this does not occur due to the presence of complement regulators like DAF and HRF. But deficiency of these factors might leads to innocent bystander lysis.
COMPLEMENT DEFICIENCY DISEASES:
Genetic deficiencies have been described for each of the complement components. Homozygous deficiencies in any of the early components of the classical pathway (C1q, C1r, C1s, C4, and C2) exhibit similar symptoms, notably a marked increase in immune-complex diseases such as systemic lupus erythematosus, glomerulonephritis, and vasculitis. But there are two important and common deficiency diseases namely hereditary angioedema and paroxysmal nocturnal hemoglobinuria.
HEREDIATARY ANGIOEDEMA:
An autosomal dominant inherited diseases called hereditary angioneurotic edema is due to a deficiency of C1 inhibitor (C1 INH). Clinical manifestations of the disease include intermittent acute accumulation of edema fluid in the skin and mucosa, which causes abdominal pain, vomiting, diarrhea and potentially life-threatening airway obstruction. In these patients, the plasma levels of C1 INH protein are sufficiently reduced that activation of C1 by immune complexes is not properly controlled and increased breakdown of C2 and C4 occurs. The mediators of edema formation in patients with hereditary angioneurotic edema include a proteolytic fragment of C2 called C2 kinin and bradykinin. C1 INH is an inhibitor of other plasma serine proteases besides C1, including kallikrein and coagulation factor XII and both activated kallikrein and factor XII can promote increased formation of bradykinin.
PAROXYSMAL NOCTURNAL HEMOGLOBINURIA:
Decay Accelerating Factor (DAF) and Homologous Restriction Factor (HRF) are glycophosphatidylinositol (GPI) linked membrane proteins expressed on endothelial cells and erythrocytes. Deficiency of an enzyme required to form such protein-lipid linkages result in failure to express many glycophosphatidylinositol-linked membrane proteins including DAF and HRF and causes a disease called paroxysmal nocturnal hemoglobinuria. This disease is characterized by recurrent bouts of intravascular hemolysis, at least partly attributable to unregulated complement activation on the surface of erythrocytes. Recurrent intravascular hemolysis in turn leads to chronic hemolytic anemia and venous thrombosis.
COMPLEMENT ESTIMATION:
Complement activity of serum is measured by estimating the highest dilution of serum lysing sheep erythrocytes sensitized by antierythrocytic antibody. Estimation of individual complement components also uses hemolytic activity in a system containing excess of all complement components except the one to be measured. Complement components can be quantitated also by radial immunodiffusion in agar but this method does not differentiate between active and inactive fractions.