COMPLEMENT FIXATION TEST
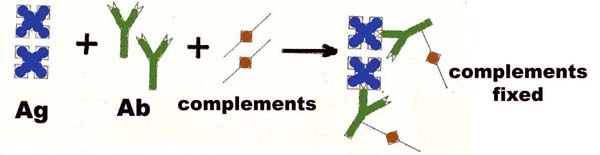
Complement fixation referred as the binding and activation of complement to Ag-Ab complex. Normally complements bind to Ag-Ab complex, i.e. antigen-antibody reactions lead to immune complex formation which produces complement fixation via the classical pathway and this may be exploited to determine the amount of antigen or antibody present. Complement fixation can detect antibody level of less than one microgram per ml.

The complement of most species will react with antibody derived from other species and guinea pig serum is a common laboratory source of complement. For most of complement fixation reaction of complement, it is difficult to identify the positive test. To overcome this effect indicator system used to identify the complement fixation. So, In complement fixation test, two system generally used, one is test system which contain sample to be tested and indicator system which signals the complement fixation which is usually an erythrocytes coated with antibodies. Complement fixation test might be used to detect the presence of either antibody or antigen.
COMPLEMENT FIXATION TEST FOR ANTIBODY DETECTION:
Test system (sample), antigens and complements are first mixed and after a period of incubation, the indicator system, and antibody coated sheep erythrocytes, are added. Finally result is observed.
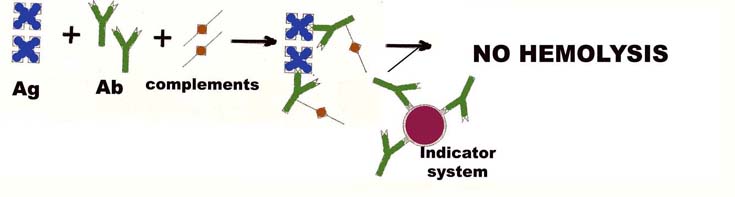
For positive test, antibody in sample binds with antigen and forms Ag-Ab complex. Then complements fixed during the incubation period and will not available to lyse the red cells. Thus a positive complement fixation test is indicated by absence of lysis of red cells.
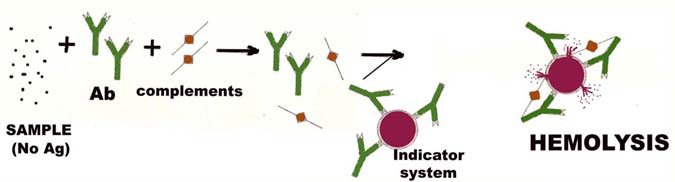
In negative test, there will no antibody for the formation of Ag-ab complex, therefore no complement fixation. Thus, the complements are free for lysing the indicator system.
CONDITIONS:
APPLICATIONS:
The classical complement fixation test is the Wassermann reaction used in the diagnosis of syphilis which is caused by Treponema pallidum. The test system consists of Wassermann antigen mixed with dilutions of the patientsí serum in the presence of guinea pig complement. After the antigen and patientsí serum have had time to react and take up the limited amount of complement available in the system, the indicator system is added to show whether or not there is a free complement. Controls are included to ensure that none of the reagents are anticomplementary and positive and negative control sera are tested in parallel. This test is also known immobilization test.
Cytolytic or cytocidal tests also are complements dependent. When a suitable live bacterium such as cholera Vibrio, is mixed with its antibody in the presence of complement, the bacterium is killed and lysed. This forms the basis of the vibriocidal antibody test for the measurement of anticholera antibodies.
Complement fixation tests are used routinely for detecting viruses in tissue cultures which have been inoculated with specimens of blood or tissue fluids from humans with probable viral infections.