CELL CYCLE
Self-reproduction is perhaps the most fundamental characteristic of cells—as may be said for all living organisms. All cells reproduce by dividing in two, with each parental cell giving rise to two daughter cells on completion of each cycle of cell division. These newly formed daughter cells can themselves grow and divide, giving rise to a new cell population formed by the growth and division of a single parental cell and its progeny. In the simplest case, such cycles of growth and division allow a single bacterium to form a colony consisting of millions of progeny cells during overnight incubation on a plate of nutrient agar medium. In a more complex case, repeated cycles of cell growth and division result in the development of a single fertilized egg into the more than 1013 cells that make up the human body.
The division of all cells must be carefully regulated and coordinated with both cell growth and DNA replication in order to ensure the formation of progeny cells containing intact genomes. In eukaryotic cells, progression through the cell cycle is controlled by a series of protein kinases that have been conserved from yeasts to mammals. In higher eukaryotes, this cell cycle machinery is itself regulated by the growth factors that control cell proliferation, allowing the division of individual cells to be coordinated with the needs of the organism as a whole. Not surprisingly, defects in cell cycle regulation are a common cause of the abnormal proliferation of cancer cells, so studies of the cell cycle and cancer have become closely interconnected, similar to the relationship between studies of cancer and the cell signaling pathways.
The Eukaryotic Cell Cycle
The division cycle of most cells consists of four coordinated processes: cell growth, DNA replication, distribution of the duplicated chromosomes to daughter cells, and cell division. In bacteria, cell growth and DNA replication take place throughout most of the cell cycle, and duplicated chromosomes are distributed to daughter cells in association with the plasma membrane. In eukaryotes, however, the cell cycle is more complex and consists of four discrete phases. Although cell growth is usually a continuous process, DNA is synthesized during only one phase of the cell cycle, and the replicated chromosomes are then distributed to daughter nuclei by a complex series of events preceding cell division. Progression between these stages of the cell cycle is controlled by a conserved regulatory apparatus, which not only coordinates the different events of the cell cycle but also links the cell cycle with extracellular signals that control cell proliferation.
Phases of the Cell Cycle
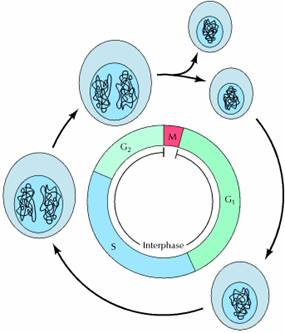
A typical eukaryotic cell cycle is illustrated by human cells in culture, which divide approximately every 24 hours. As viewed in the microscope, the cell cycle is divided into two basic parts: mitosis and interphase. Mitosis (nuclear division) is the most dramatic stage of the cell cycle, corresponding to the separation of daughter chromosomes and usually ending with cell division (cytokinesis). However, mitosis and cytokinesis last only about an hour, so approximately 95% of the cell cycle is spent in interphase—the period between mitoses. During interphase, the chromosomes are decondensed and distributed throughout the nucleus, so the nucleus appears morphologically uniform. At the molecular level, however, interphase is the time during which both cell growth and DNA replication occur in an orderly manner in preparation for cell division.
The cell grows at a steady rate throughout interphase, with most dividing cells doubling in size between one mitosis and the next. In contrast, DNA is synthesized during only a portion of interphase. The timing of DNA synthesis thus divides the cycle of eukaryotic cells into four discrete phases. The M phase of the cycle corresponds to mitosis, which is usually followed by cytokinesis. This phase is followed by the G1 phase (gap 1), which corresponds to the interval (gap) between mitosis and initiation of DNA replication. During G1, the cell is metabolically active and continuously grows but does not replicate its DNA. G1 is followed by S phase (synthesis), during which DNA replication takes place. The completion of DNA synthesis is followed by the G2 phase (gap 2), during which cell growth continues and proteins are synthesized in preparation for mitosis.

The duration of these cell cycle phases varies considerably in different kinds of cells. For a typical rapidly proliferating human cell with a total cycle time of 24 hours, the G1 phase might last about 11 hours, S phase about 8 hours, G2 about 4 hours, and M about 1 hour. Other types of cells, however, can divide much more rapidly. Budding yeasts, for example, can progress through all four stages of the cell cycle in only about 90 minutes. Even shorter cell cycles (30 minutes or less) occur in early embryo cells shortly after fertilization of the egg. In this case, however, cell growth does not take place. Instead, these early embryonic cell cycles rapidly divide the egg cytoplasm into smaller cells. There is no G1 or G2 phase, and DNA replication occurs very rapidly in these early embryonic cell cycles, which therefore consist of very short S phases alternating with M phases.
In contrast to the rapid proliferation of embryonic cells, some cells in adult animals cease division altogether (e.g., nerve cells) and many other cells divide only occasionally, as needed to replace cells that have been lost because of injury or cell death. Cells of the latter type include skin fibroblasts, as well as the cells of many internal organs, such as the liver, kidney, and lung. As discussed further in the next section, these cells exit G1 to enter a quiescent stage of the cycle called G0, where they remain metabolically active but no longer proliferate unless called on to do so by appropriate extracellular signals.
Analysis of the cell cycle requires identification of cells at the different stages discussed above. Although mitotic cells can be distinguished microscopically, cells in other phases of the cycle (G1, S, and G2) must be identified by biochemical criteria. Cells in S phase can be readily identified because they incorporate radioactive thymidine, which is used exclusively for DNA synthesis. For example, if a population of rapidly proliferating human cells in culture is exposed to radioactive thymidine for a short period of time (e.g., 15 minutes) and then analyzed by autoradiography, about a third of the cells will be found to be radioactively labeled, corresponding to the fraction of cells in S phase.
Variations of such cell labeling experiments can also be used to determine the length of different stages of the cell cycle. For example, consider an experiment in which cells are exposed to radioactive thymidine for 15 minutes, after which the radioactive thymidine is removed and the cells are cultured for varying lengths of time prior to autoradiography. Radioactively labeled interphase cells that were in S phase during the time of exposure to radioactive thymidine will be observed for several hours as they progress through the remainder of S and G2. In contrast, radioactively labeled mitotic cells will not be observed until 4 hours after labeling. This 4-hour lag time corresponds to the length of G2—the minimum time required for a cell that incorporated radioactive thymidine at the end of S phase to enter mitosis.
Cells at different stages of the cell cycle can also be distinguished by their DNA content . For example, animal cells in G1 are diploid (containing two copies of each chromosome), so their DNA content is referred to as 2n (n designates the haploid DNA content of the genome). During S phase, replication increases the DNA content of the cell from 2n to 4n, so cells in S have DNA contents ranging from 2n to 4n. DNA content then remains at 4n for cells in G2 and M, decreasing to 2n after cytokinesis. Experimentally, cellular DNA content can be determined by incubation of cells with a fluorescent dye that binds to DNA, followed by analysis of the fluorescence intensity of individual cells in a flow cytometer or fluorescence-activated cell sorter, thereby distinguishing cells in the G1, S, and G2/M phases of the cell cycle.
Regulation of the Cell Cycle by Cell Growth and Extracellular Signals
The progression of cells through the division cycle is regulated by extracellular signals from the environment, as well as by internal signals that monitor and coordinate the various processes that take place during different cell cycle phases. An example of cell cycle regulation by extracellular signals is provided by the effect of growth factors on animal cell proliferation. In addition, different cellular processes, such as cell growth, DNA replication, and mitosis, all must be coordinated during cell cycle progression. This is accomplished by a series of control points that regulate progression through various phases of the cell cycle.
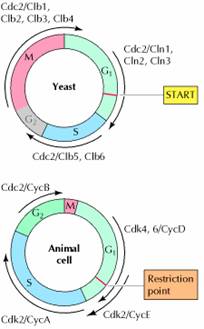
A major cell cycle regulatory point in many types of cells occurs late in G1 and controls progression from G1 to S. This regulatory point was first defined by studies of budding yeast (Saccharomyces cerevisiae), where it is known as START . Once cells have passed START, they are committed to entering S phase and undergoing one cell division cycle. However, passage through START is a highly regulated event in the yeast cell cycle, where it is controlled by external signals, such as the availability of nutrients, as well as by cell size. For example, if yeasts are faced with a shortage of nutrients, they arrest their cell cycle at START and enter a resting state rather than proceeding to S phase. Thus, START represents a decision point at which the cell determines whether sufficient nutrients are available to support progression through the rest of the division cycle. Polypeptide factors that signal yeast mating also arrest the cell cycle at START, allowing haploid yeast cells to fuse with one another instead of progressing to S phase.
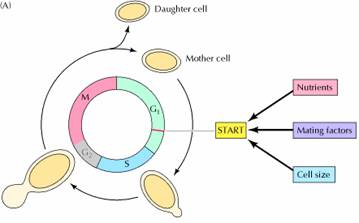
In addition to serving as a decision point for monitoring extracellular signals, START is the point at which cell growth is coordinated with DNA replication and cell division. The importance of this regulation is particularly evident in budding yeasts, in which cell division produces progeny cells of very different sizes: a large mother cell and a small daughter cell. In order for yeast cells to maintain a constant size, the small daughter cell must grow more than the large mother cell does before they divide again. Thus, cell size must be monitored in order to coordinate cell growth with other cell cycle events. This regulation is accomplished by a control mechanism that requires each cell to reach a minimum size before it can pass START. Consequently, the small daughter cell spends a longer time in G1 and grows more than the mother cell. The proliferation of most animal cells is similarly regulated in the G1 phase of the cell cycle. In particular, a decision point in late G1, called the restriction point in animal cells, functions analogously to START in yeasts. In contrast to yeasts, however, the passage of animal cells through the cell cycle is regulated primarily by the extracellular growth factors that signal cell proliferation, rather than by the availability of nutrients. In the presence of the appropriate growth factors, cells pass the restriction point and enter S phase. Once it has passed through the restriction point, the cell is committed to proceed through S phase and the rest of the cell cycle, even in the absence of further growth factor stimulation. On the other hand, if appropriate growth factors are not available in G1, progression through the cell cycle stops at the restriction point. Such arrested cells then enter a quiescent stage of the cell cycle called G0, in which they can remain for long periods of time without proliferating. G0 cells are metabolically active, although they cease growth and have reduced rates of protein synthesis. As already noted, many cells in animals remain in G0 unless called on to proliferate by appropriate growth factors or other extracellular signals. For example, skin fibroblasts are arrested in G0 until they are stimulated to divide as required to repair damage resulting from a wound. The proliferation of these cells is triggered by platelet-derived growth factor, which is released from blood platelets during clotting and signals the proliferation of fibroblasts in the vicinity of the injured tissue.
Although the proliferation of most cells is regulated primarily in G1, some cell cycles are instead controlled principally in G2. One example is the cell cycle of the fission yeast Schizosaccharomyces pombe. In contrast to Saccharomyces cerevisiae, the cell cycle of S. pombe is regulated primarily by control of the transition from G2 to M, which is the principal point at which cell size and nutrient availability are monitored. In animals, the primary example of cell cycle control in G2 is provided by oocytes. Vertebrate oocytes can remain arrested in G2 for long periods of time (several decades in humans) until their progression to M phase is triggered by hormonal stimulation. Extracellular signals can thus control cell proliferation by regulating progression from the G2 to M as well as the G1 to S phases of the cell cycle.
Multicellular organisms such as plants and animals are composed of millions to a trillion (1,000,000,000) cells that work together. The cells that make up different tissues have different shapes and perform different functions for the plant or animal. Even though they have diverse functions, each somatic cell in the organism normally has the same chromosomes and therefore the same genetic makeup. Furthermore, the millions of cells that makeup a mature organism originated from a single cell formed when the male and female gametes from the parents of the organism fused. This single cell established the life of the organism. Understanding multicellular organisms requires an understanding of the lifecycle of the cells that make up the organism.
Let's think about the cell cycle from a personal point of view. Your age plus about nine months ago you were a zygote, a single cell formed when the sperm and egg from your biological parents fused in a fallopian tube (or possibly in a test tube if in vitro fertilization factored into your birth). You have come a long way since then, progressing one cell cycle at a time. The cell cycle is the life cycle of a single cell. We depict the cell cycle in a circular diagram although the cells in your body do not actually go around in circles. The main idea is that when new cells are made from existing cells, the new cells start their lifecycle and the old cells end theirs.
|
|
|
Fig. 1: The cell cycle depicts the stages in the life of a cell. |
The first part of the cell cycle is the G1 phase. Cells can go through
growth and development in this phase. Some cells differentiate into specialized
cells and then never leave this phase. However, a zygote doesn't grow in size,
but instead continues the cell cycle so it can quickly give rise to more cells.
The second part of the cell cycle is the S phase (synthesis phase). Here, the
cell replicates it's chromosomes so that it has a copy of each chromosome to
pass on to daughter cells. The third part of the cell cycle is the G2 phase
where the cell prepares for division. The G1, S and G2 phases together are
called interphase. The M phase completes the cell cycle. 'M' could be mitosis or
meiosis depending on the type of cell. For the zygote, the goal is to make more
somatic cells. Therefore it goes through mitosis and gives rise to two daughter
cells. This completes the life cycle of the zygote and starts the lifecycle of
the new cells. Rounds of the cell cycle continued over and over to form the body
you have today. As long as you live, some of your cells must be able to complete
the cell cycle.
From a cytogenetics point of view, you have two types of cells in your body. You
have cells with 46 chromosomes (somatic or body cells) and cells with 23
chromosomes (gamete or sex cells). Since you started as a single cell with 46
chromosomes, there must be two types of cell division taking place in your body
to accommodate both somatic and gamete cells. Mitosis and meiosis are the two
types of cell division.
|
|
|
Fig. 2: Multicellular organisms that sexually reproduce have diploid and haploid cells. |
MITOSIS:
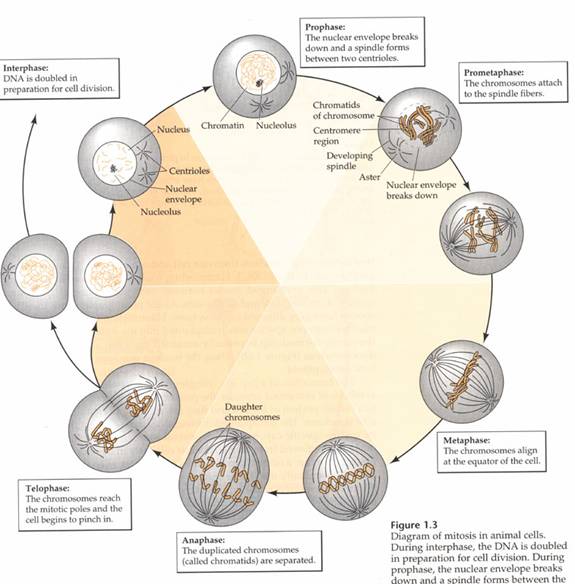
The objective of mitosis is to make two genetically identical cells from a single cell. In the cells of our body, we start with 46 chromosomes in a single cell and end up with 46 chromosomes in two cells. Obviously, replicating the chromosomes is a prerequisite to mitosis. Remember, replication takes place during interphase when the chromosomes are dispersed structures in the nucleus. Mitosis is an organized procession of activity in the cell that allows the replicated chromosomes to be properly divided into two identical cells. Chromosomes are important because they contain genes. Pictures depict the four stages of mitosis. The events at each stage that are important in understanding the distribution of genes during cell division will be described.
Mitosis: Prophase
Prophase is the beginning of mitosis (Fig. 3). During interphase, the chromosomes look like a plate of spagetti in the nucleus. It is difficult to pick out an individual chromosome because they are each so spread out. The chromosomes in the nucleus change from being loosely dispersed to becoming more condensed. This change in chromosome structure makes them easier to move around the cell, an important issue for what is about to happen.
|
|
|
|
|
|
Fig. 3 and 4 - Prophase and metaphase of mitosis
As the chromosomes condense they get shorter and thicker and can be seen through the microscope as individual structures. The chromosomes at prophase will consist of two identical parts called sister chromatids that stay connected at the centromere. It is now clear that the chromosomes have been replicated. Next, the nucleus is dissolved. At the end of prophase the replicated chromosomes are moved by the spindle apparatus to the center of the cell.
Mitosis: Metaphase
When the chromosomes are maneuvered to the center of the cell, metaphase begins (fig. 4). The spindle fiber network connects the centromere of the replicated chromosome to the outer part of each cell. The chromosomes now resemble the line of scrimmage of a football team, poised and waiting for some cellular signal to begin the next phase.
Mitosis: Anaphase
At anaphase (Fig. 5) the chromatids making up each chromosome are pulled apart and begin to move away from each other under the control of the spindle fibers. Once they are pulled apart, the chromatids are now considered individual chromosomes. As anaphase progresses, the chromosomes on each end of the cell are pulled into a bundle. When they stop moving, telophase begins.
|
|
|
Fig. 5: Anaphase of mitosis. |
Mitosis: Telophase
|
|
|
Fig. 6: Telophase of
Mitosis.
|
The final stage of mitosis is telophase (Fig. 6). A nuclear envelope will form around each bundle of chromosomes. The cell will undergo cytokinesis and the cytoplasm is split between the two identical daughter cells. Cell membranes (and cell walls in plants) form between the two cells. The chromosomes begin to decondense. They loosen up and spread around the nucleus because they no longer will need to move around. The new cells have now begun their lifecycle.
MEIOSIS:
The objective of meiosis is to make four cells from a single somatic cell. The four cells each have half the chromosome number found in the somatic cell. In our human bodies, the four gametes will each have 23 chromosomes which means the 46 chromosomes in the somatic cell must replicate during interphase prior to meiosis just as they would before mitosis. Meiosis occurs in specialized cells of the body called germline cells.
To appreciate meiosis and gamete formation it is important to first understand two ideas, chromosome sets and homologous chromosomes.
Chromosome sets: The 46 chromosomes you have consist of two sets. You are a diploid organism ('di' means two and 'ploid' means sets). One set of chromosomes came from each parent when their gametes fused. Therefore, human gametes are haploid (one set).
Homologous chromosomes: The 46 chromosomes in a somatic cell can be arranged into 23 homologous or similar pairs. One chromosome from each pair came from the male parent, the other from the female. Homologous chromosomes have the same genes although in heterozygous people the genes would be different alleles (A,a). The exception to this would be the sex (X and Y) chromosomes. Passing on a complete set of human genes requires one chromosome from each pair to end up in each gamete.
The key events that happen in each of the stages of meiosis are summarized.
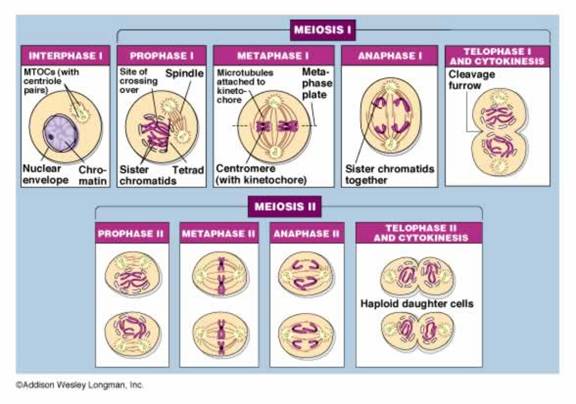
Meiosis: Prophase I
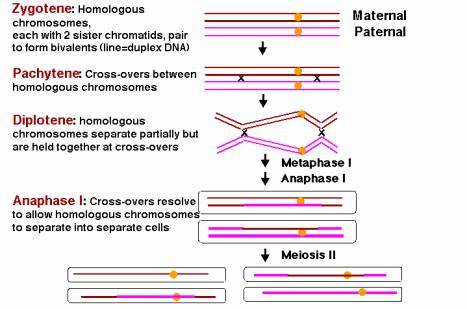
This stage starts meiosis and is the same as prophase of mitosis with one important change. As the chromosomes condense, they form groups of four chromatids called tetrads or bivalents. Close inspection reveals that each chromosome is replicated and consists of two sister chromatids. The two chromosomes in each cell that are homologous and have the same genes (but perhaps different alleles if the organism is heterozygous) will pair closely. This close association, or synapsis, allows the homologous chromosomes to crossover and exchange identical parts. The impact of crossing over is that genes that are on the same chromosome (ie. D and B in Fig.7) can be recombined so that they are not always inherited together. The tetrad or bilvalent formed during synapsis remains assembled as prophase progresses. The tetrad therefore moves as a unit to the center of the cell.
|
|
|
|
|
|
Fig. 7 and 8. Prophase l and metaphase l of meiosis
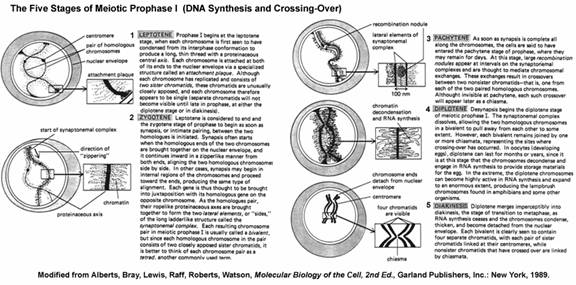
Prophase I occurs in five substages namely Leptotene, zygotene, Pachytene, Diplotene and Diakinensis.
Leptotne:
Prophase-I begins at the leptotene stage, when each chromosome is first seen to have condensed from its interphase conformation to produce a long, thin thread with a proteinaceous central axis. Each chromosome is attached at both of its ends to the nuclear envelope via a specialized structure called an attachment plaque. Although each chromosome has replicated and consists of two sister chromatids, these chromatids are unusually closely apposed, and each chromosome therefore appears to be single (separate chromatids will not become visible until late in prophase, at either the diplotene stage or in diakinesis.
Zygotene:
Leptotene is considered to end and the zygotene stage of prophase to begin as soon as synapsis, or intimate pairing, between the two homologous is initiated. Synapsis often starts when the homologous ends of the two chromosomes are brought together on the nuclear envelope and it continues inward in a zipperlike manner from both ends, aligning the two homologous chromosomes side by side. In other cases, synapsis may begin in internal regions of the chromosomes and proceed toward the ends, producing the same type of alignment. Each gene is thus thought to the brought into juxtaposition with its homologous gene on the opposite chromosome. As the homologues pair, their ropelike proteinaceous axes are brought together to form the two lateral elements, or “sides,” of the long ladderlike structure called the synaptonemal complex. Each resulting chromosome pair in meiotic prophase I is usually called a bivalent, but since each homologous chromosome in the pair consists of two closely apposed sister chromatids, it is better to think of each chromosome pair as a tetrad, another commonly used term.
Pachytene:
As soon as synapsis is complete all along the chromosomes, the cells are said to have entered the pachytene stage of prophase, where they may remain for days. At this stage, large recombination nodules appear at intervals on the synaptonemal complexes and are thought to mediate chromosomal exchanges. These exchanges result in crossovers between two non-sister chromatids-that is, one from each of the two paired homologous chromosomes. Although invisible at pachytene, each such crossover will appear later as a chiasma.
Diplotene:
Desynapsis begins the diplotene stage of meiotic prophase I. The synaptonemal complex dissolves, allowing the two homologous chromosomes in a bivalent to pull away from each other to some extent. However, each bivalent remains joined by one or more chiasmata, representing the sites where crossing over has occurred. In oocytes (developing eggs), diplotene can last for months or years, since it is at this stage that the chromosomes decondense and engage in RNA synthesis to provide storage materials for the egg. In the extreme, the diplotene chromosomes can become highly active in RNA synthesis and expand to an enormous extent, producing the lampbrush chromosomes found in amphibians and some other organisms.
Diakinesis:
Diplotene merges imperceptibly into diakinesis, the stage of transition to metaphases, as RNA synthesis ceases and the chromosomes condense, thicken and become detached from the nuclear envelope. Each bivalent is clearly seen to contain four separate chromatids, with each pair of sister chromatids linked at their centromeres, while non-sister chromatids that have crossed over are linked by chiasmata.
Meiosis: Metaphase I
Metaphase-I starts when the tetrads are at the center of the cell (Fig. 8). The tetrads have stayed together which insures that during the first division, each cell will get one chromosome from each homologous pair. The chromosomes remain at the center of the cell until the homologous pairs are ready to move away from each other.
|
|
|
Fig. 8: Metaphase I of meiosis. |
Meiosis: Anaphase I
The chromosomes that make up each tetrad
separate during anaphase I.
However, the sister chromatids will stay connected at the centromere. Anaphase I
proceeds until the chromosomes are pulled into a bundle at opposite ends of the
cell.
|
|
|
|
|
|
Fig. 9 and 10. Anaphase l and telophase l of meiosis.
Meiosis: Telophase l
|
|
|
Fig. 10: Telophase l of meiosis. |
The cell divides into two cells during telophase I (Fig. 10). The bundle of chromosomes may have a nuclear envelope develop around them. The germline cells in some organisms such as human females, go through the first four stages of meiosis prior to birth. The germline cells remain at telophase I for some time. The second round of division occurs when the gamete is needed for reproduction. In other situations, telophase I is an abbreviated stage and the second round of division proceeds without delay.
Meiosis: Prophase II
If the chromosomes became dispersed in telophase I they will condense again at prophase II. The spindle apparatus moves the chromosomes to the middle of the cell. Look! The centromeres are still holding the sister chromatids together (Fig. 11).
|
|
|
|
|
|
Fig. 11 and 12. Prophase II and metaphase II of meiosis.
Meiosis: Metaphase II
In metaphase II the chromosomes are aligned at the center of the cell (Fig. 12). This time there are not homologous chromosomes to be paired with. This metaphase looks similar to metaphase of mitosis but there is a key difference. What is the difference? (Compare Figs. 4 and 12).
|
|
|
|
|
|
Fig. 4 and 12. Metaphase of mitosis and metaphase II of meiosis.
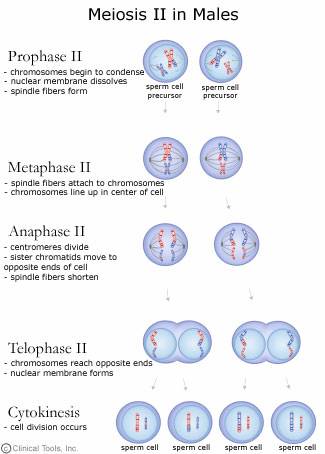
Meiosis: Anaphase II
During anaphase II, the chromatids are pulled apart by the spindle fibers. Now they are classified as chromosomes, not chromatids. The chromosomes move apart to opposite ends of the cell (Fig. 13).
|
|
|
|
|
|
Fig. 13 and 14. Anaphase II and telophase II of meiosis.
Meiosis: Telophase II
In the final stage of meiosis, telophase II, the nucleus forms around the bundle of chromosomes (Fig. 14). The cell divides. Now four cells exist that originated from one germline cell. Each cell is a gamete with half the number of chromosomes and genes as a somatic cell.
|
|
|
Fig. 14: Telophase II of meiosis. |
Parallel Behavior and the Chromosome Theory
The successful completion of mitosis or meiosis requires the cell to move large objects with precision and control many detailed events. The process surpasses any engineering accomplishments of NASA. The importance of mitosis and meiosis to an organism is obvious when we consider that genes are a part of the chromosome and the genes must be copied and distributed properly to produce viable daughter cells. The mechanisms of these events are far from being completely understood. From our current understanding we can appreciate how the principles of segregation and independent assortment are controlled by the mechanics of meiosis.
When cell division was
first observed and described by cytogeneticists, biologists were just beginning
to accept the idea that genes were tiny objects that controlled traits and
existed in the cells of living things. Two biologists, a German named Boveri and
an American graduate student named Sutton, recognized that chromosome behavior
during meiosis matched Mendel's principles of gene behavior. Both scientists
proposed the idea that while genes had not yet been directly observed, they must
be a part of the chromosome. Sutton and Boveri are both given credit for
proposing this chromosome theory; genes are a part of chromosomes.
Segregation predicts gene behavior that matches the chromosome behavior observed
by cytogeneticists. Genes are in pairs because chromosomes are in pairs. The
gene pairs associate during gamete formation when the homologous chromosomes
pair in prophase I. The gene pairs separate when the homologous pairs seperate
in anaphase I followed by chromatid separation in anaphase II. Thus chromosome
behavior dictates the gene's segregation behavior.
Independent assortment of gene pairs also correlates with chromosome behavior.
Lets consider our dihybrid individual with the genotype BbEe (Fig. 15). How many
kinds of gametes can it make? The four possible combinations (BE, bE, Be and be)
are made at equal frequencies. We can understand why this occurs if we think
about what happens to chromosomes at metaphase I of meiosis. The chromosome pair
that carries the 'E' and 'e' genes will be moved independently from the
chromosome pair with the 'B' and 'b' chromosomes. When the chromosomes arrive at
the center of the cell at metaphase I, the two tetrads may be aligned so that
the 'E' and 'B' genes move to one cell and the 'e' and 'b' genes move to the
other during the first division. In the other half of the cells that go through
meiosis, the tetrads will line up so that the 'E' genes will be passed on with
the 'b' genes and the 'e' genes will go with the 'B' genes. Keep in mind that
organisms that make gametes make thousands of them. The chance alignment of the
tetrads at metaphase I therefore dictates the overall frequency of gametes with
different combinations of genes when the genes are on separate chromosomes.
|
|
|
|
Fig. 15: Alignment of chromosomes during metaphase I will determine the gene combinations that segregate into gametes.
When more gene pairs are considered, the same scenarios described above will be true as long as the genes are on separate chromosomes. When genes are on the same chromosome, the role of crossing over on gene inheritance needs to be considered in more detail. We will cover the inheritance of genes on the same chromosome in a later lesson.
There are several key differences between meiosis and mitosis that are summarized in the following table:
|
Mitosis |
Meiosis |
|
Chromosome number stays the same |
Chromosome number is halved |
|
One division occurs to make two cells. Four stages of this division. |
Two divisions occur to make four cells. Eight stages in these divisions. |
|
Similar or homologous chromosomes do not pair. |
Homologous chromosomes pair during prophase l. Pairing is called synapsis. |
|
Crossover exchanges between homologous chromosomes is rare. |
Synapsis allows crossing over between homologous chromosomes. |
|
Two cells made are genetically identical. |
Four cells made are genetically |